-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(2): 47-54
doi:10.5923/j.chemistry.20160602.04

Adsorption of Methylene Blue on the Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5, 1) Lyonsite Phases
Khadija Gourai 1, Abdeslam El Bouari 1, Bouchra Belhorma 2, Lahcen Bih 3
1Department of Chemistry, Laboratory of Physical Chemistry of Applied Materials, Faculty of Sciences Ben M’Sik, University Hassan II Casablanca, Casablanca, Morocco
2National Center for Energy, Nuclear Science and Technology, Rabat, Morocco
3Team Physical Chemistry of Condensed Matter, Faculty of Sciences Meknes, University Moulay Ismail, Meknes, Morocco
Correspondence to: Khadija Gourai , Department of Chemistry, Laboratory of Physical Chemistry of Applied Materials, Faculty of Sciences Ben M’Sik, University Hassan II Casablanca, Casablanca, Morocco.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work focuses on the study of the adsorption of methylene blue (MB) onto the mixed transition metal oxide compounds Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) of Lyonsite structure. The studies are carried out in batch methods, and they allowed determining several parameters that govern this adsorption. The influence of pH, temperature, contact time and mass of the adsorbent on the MB adsorption were determined. The adsorption kinetics was analyzed using the Langmuir and Freundlich models. The obtained results show that the adsorption of methylene blue onto these mixed oxides is well described by the model of Langmuir and its kinetic corresponds to the second order.
Keywords: Adsorption, Methylene blue, Kinetic, Isotherm, Lyonsite structure
Cite this paper: Khadija Gourai , Abdeslam El Bouari , Bouchra Belhorma , Lahcen Bih , Adsorption of Methylene Blue on the Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5, 1) Lyonsite Phases, American Journal of Chemistry, Vol. 6 No. 2, 2016, pp. 47-54. doi: 10.5923/j.chemistry.20160602.04.
Article Outline
1. Introduction
- The treatment of brackish water by reverse osmosis is a membrane filtration technique which allows production of good quality water for different applications (drinking water, agriculture, industries...) [1, 2]. This treatment requires a series of meadows-treatments such as the filtration with activated carbon, which is based on the phenomenon of adsorption, and is relatively easy to implement. The activated carbon is the most widely used adsorbent because of its high adsorption capacity [3]; but it has the disadvantage of a high cost and difficulty to regenerate after each adsorption cycle which limits its commercial application [4]. Hence, several investigations looking for new adsorbents with high efficiency and low cost are performed [5-7]. In order to evaluate how these adsorbents are more effective, the studies considered the investigation of the mechanism of attachment of dye molecules on the surface of the adsorbents.According to the literature [8, 9], several new adsorbents based on transition metal oxide were proposed. In the framework of looking for new adsorbent materials, our interest is focused on oxide compounds based on molybdate Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) containing transition metals like iron and chromium. In fact, the presence of these transition metals in the structure of these molybdates could facilitate the adsorption and therefore the elimination of impurities from water. The first molybdate material which has the lyonsite structure is the compound NaCo2.31(MoO4)3 [10]. In general, oxides of lyonsite structure can be expressed as A16B12O48 and are composed of an assembly of octahedra AO6 and tetrahedrons BO4. The octahedral sites AO6 are occupied by heavily loaded cations of electrical-charge +5 and +6 and the tetrahedral sites BO4 are occupied by the cations of charges +1, +2 and +3 such as (Li+, K+, Na+, Zn2+, Ca2+… ) [11].In this work, we present first the method of preparation of the molybdates Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) and then the studies of adsorption of methylene blue onto these compounds. The influence of pH, temperature, contact time and mass of the adsorbent on the adsorption capacity is studied. The adsorption kinetic is determined using the models of Langmuir and Freundlich.
2. Materials and Methods
2.1. Adsorbent
- The adsorbents used in this study are the molybdates Lyonsite type of the formulae Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1). The powders of Li3Fe1-xCrx(MoO4)3 are prepared by the soft combustion method which consists to dissolve in a minimum volume of water a mixture of glycine (C2H5NO2)2 and stoichiometric reagents nitrates (LiNO3), Fe(NO3)3.9H2O, Cr(NO3)3.9H2O and (NH4)6Mo7O24.4H2O). These solutions provide highly viscous liquids after dehydration at a temperature of 80°C. Then a heat treatment between 150°C and 200°C allows to obtain powders of porous molybdates Li3Fe1-xCrx(MoO4)3. Finally, these powders were submitted to a final heat treatment at 700°C. The structural analysis of these samples was carried out by X-ray diffraction (XRD) technique using a Bruker D8 Advance apparatus. The UV-Visible spectra of the solutions are measured by a UV-3100PC spectrophotometer VWR. A pH-meter PHSJ-5 was used for the pH measurements.
2.2. Adsorbate
- The adsorbate considered in this work is the 3.7-bis-(dimethylamino) phenazathionium known as the methylene blue which is a cationic dye, and it was synthesized the first time by Heinrich Caro in 1876. Its molecular formula is C16H18CIN3S, with a molar mass equal to 319.852 g/mol and a pH equal to 6.5. The Fig.1 shows the chemical structure of methylene blue.
 | Figure 1. Chemical Structure of methylene blue |
2.3. Adsorption Experiments
- The adsorption was made in batch method and the concentration of the solution was determined by using UV–visible spectrophotometer (VWR, V 3100). The calculation of the adsorption capacity is carried out from the following equation:
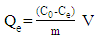 | (1) |
3. Results and Discussion
3.1. X-Ray Diffraction Analysis
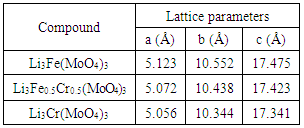
- X-ray patterns of Li3Fe1-xCrx(MoO4)3 (x= 0, 0.5 and 1) samples are shown in Fig.2. The replacement of iron (Fe3+) by chromium (Cr3+) gives X-ray patterns with the same shapes but with a small displacement of X-ray peaks. It is demonstrated that the substitution of iron by chromium form a continuous solid solution because Cr3+ (r = 0.615 Å) and Fe3+ (r = 0.645 Å) [12] have similar sizes. The results show that these materials crystalize in the orthorhombic pattern, with a space group Pnma. The experimental XRD data for all the samples are successfully indexed by DICVOL software and the linear cell parameters are gathered in Table 1. It is noticed that the substitution of iron by chromium induces the decrease of these parameters owing to the fact that the ionic radius of chromium Cr3+ is slightly smaller compared to that of Fe3+.
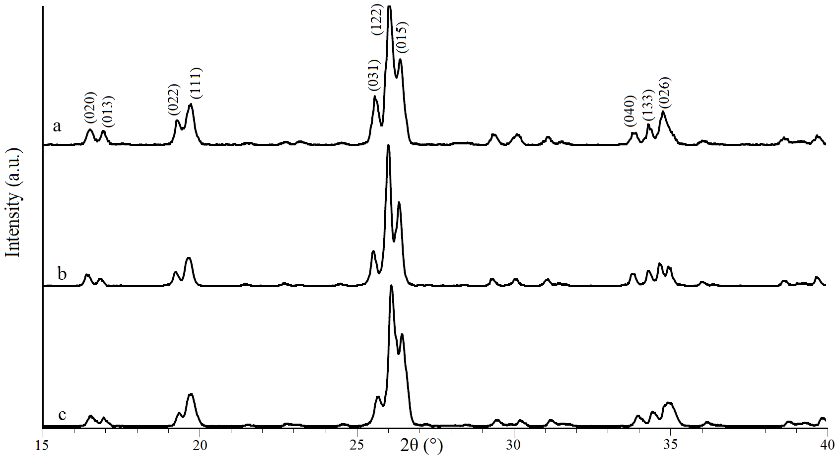 | Figure 2. XRD patterns of Li3Fe1-xCrx(MoO4)3 : (a) x = 0, (b) x = 0.5 and (c) x = 1 |
|
3.2. Influence of Some Parameters on the Adsorption
3.2.1. Effect of Contact time
- The effect of contact time on adsorption of MB onto the Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) samples is studied. The experiments were realized at room temperature by keeping the pH of the solution constant at 6.5 and using 20 mg/l MB. Fig.3 showed the obtained results. The uptake of MB onto the different adsorbents is nearly the same. The adsorption equilibrium is reached in 5 min (28 mg/g), and the adsorption capacity becomes nearly constant without changes after this equilibrium time. The short equilibrium time (5 min) could indicate that the adsorption reaction between MB and lyonsite samples is very fast [13].
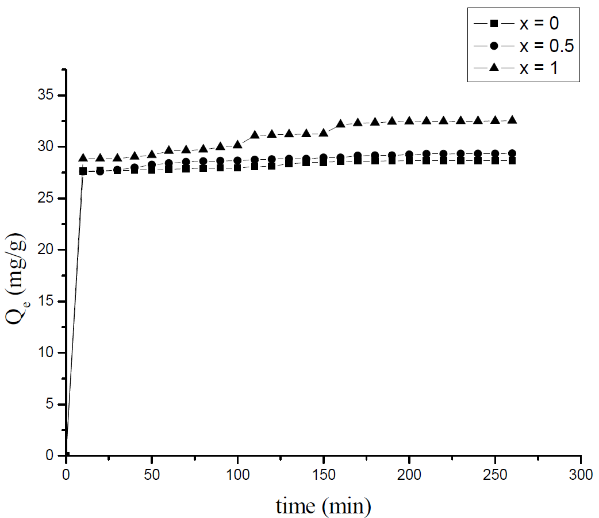 | Figure 3. Influence of the contact time on the adsorption of the MB on Li3Fe1-xCrx(MoO4)3 |
3.2.2. Effect of Temperature
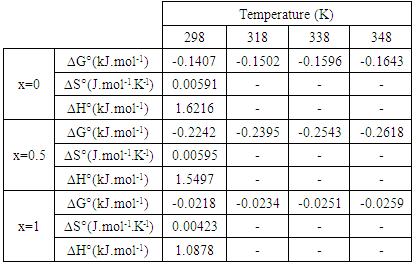
- In general, the adsorption depends on the structure of the adsorbent and on the temperature. To get an idea of this dependency, we studied the effect of temperature on the adsorption of MB onto the prepared molybdate adsorbents. The experiments were performed by adding 20 mg of Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) to a solution of 100 ml of methylene blue at different temperature. The temperatures were set at 25°C, 45°C, 65°C and 75°C using a thermostat bath. Fig.4 shows the dependence of the adsorption content of MB on the temperature.
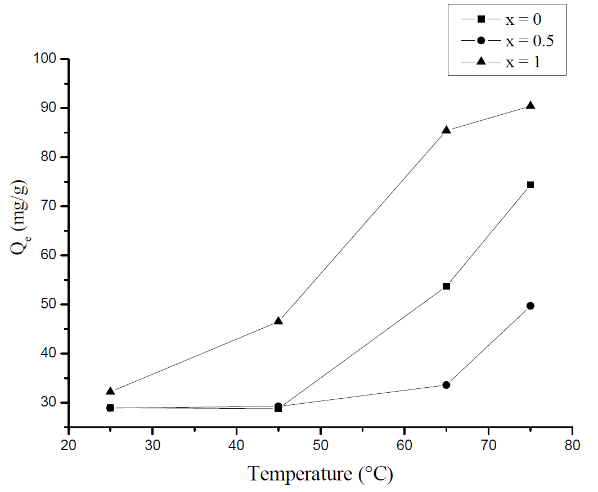 | Figure 4. The temperature dependence of the MB adsorption |
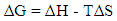 | (2) |
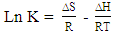 | (3) |
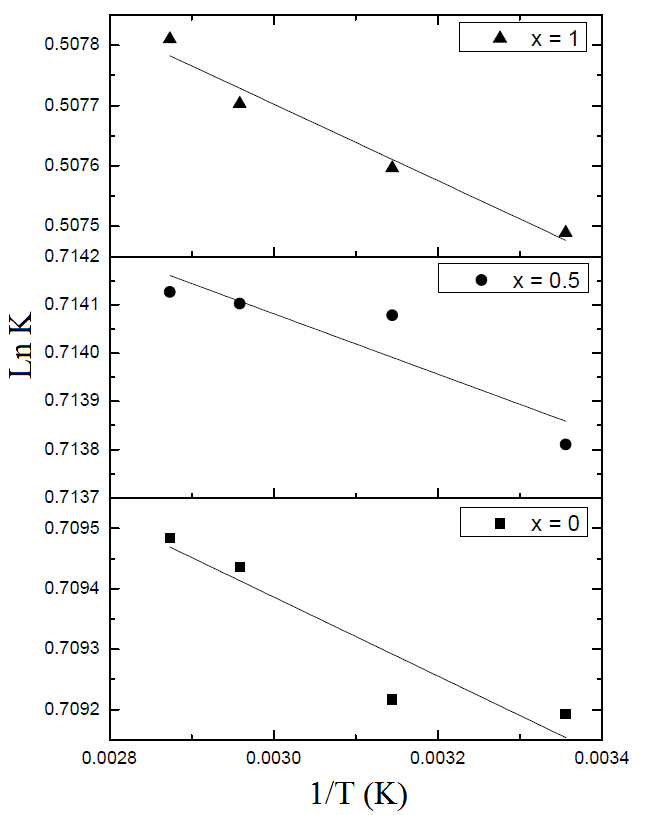 | Figure 5. Variation of the equilibrium constant Ln K as a function of temperature for MB adsorption on Li3Fe1-xCrx(MoO4)3 |
|
3.2.3. Effect of pH
- The pH is an important factor in any study of adsorption because it can influence at the same time the adsorbent structure and the mechanism of the adsorption [19]. The effect of the pH on the adsorption is studied by adjusting the initial pH of the methylene blue, by adding NaOH and HCl, to adjust the pH to 2, 4, 6, 8, and 10. The experiments were performed by adding 20 mg of Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) to 100 ml of the methylene blue solution (20 mg/l) at different pH values under the temperature of 25°C.The effect of the pH of the aqueous solution in the MB adsorption onto the Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) can be attributed to the variation in the degree of ionization of MB and the surface properties of the molybdates. The influence of pH on the adsorption is demonstrated in Fig.6. This latter indicates that an increase in solution pH from 2 to 10 induces an increase in the MB adsorption capacity on molybdate adsorbents. It is observed that the MB adsorption is pH-dependent and the uptake of the MB onto the molybdate adsorbents is favorable at higher pH value. For instance, at low pH values (from 2 to 4), the adsorbed quantity is about 26 mg/g; by increasing pH value up to 6 the uptake quantity becomes 34 mg/g. At high pH values the adsorbed quantity attains 93.8 mg/g [20].
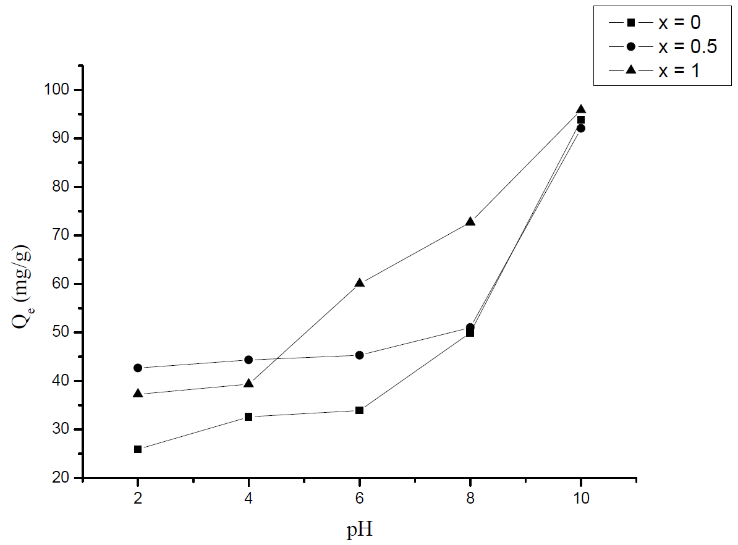 | Figure 6. Influence of pH on the adsorption of MB on Li3Fe1-xCrx(MoO4)3 |
3.2.4. Effect of Adsorbent Mass
- The effect of mass is studied by adding 100 ml of 20 mg/l MB to different masses of the lyonsite adsorbent 20, 40, 60, 80 and 100 mg. The experiment is done at room temperature at pH = 6.5. Fig.7 shows the effect of adsorbent mass on MB adsorption by molybdate lyonsite adsorbent.
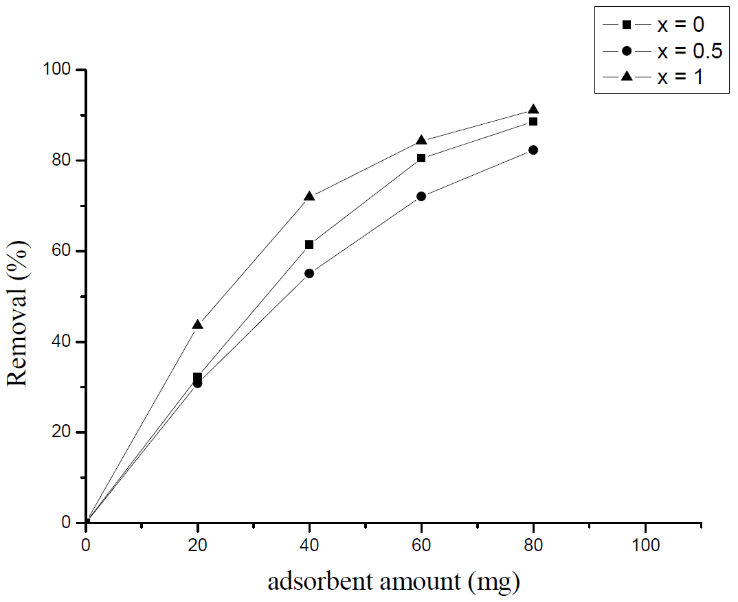 | Figure 7. Effect of adsorbent mass on MB adsorption onto Li3Fe1-xCrx(MoO4)3 phases |
3.3. Adsorption Kinetics
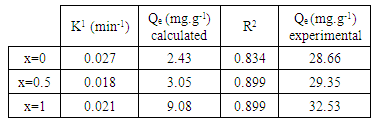
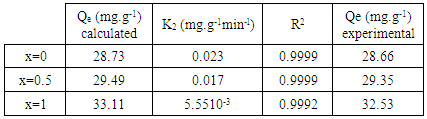
- The adsorption kinetic is an essential issue in the adsorption studies since it reveals some aspects of the adsorption process. The study of adsorption kinetics of MB onto Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) molybdates is considered in the framework of the first-order and the second-order models. The conformity between the experimental data obtained from the kinetic experiments and the model prediction are based on the values of the correlation coefficients (R2); the value of R2 nearest to the unit indicates the proper model to describe correctly the adsorption kinetics.The linear forms of these models are given by the following equations:
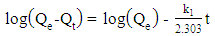 | (4) |
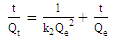 | (5) |
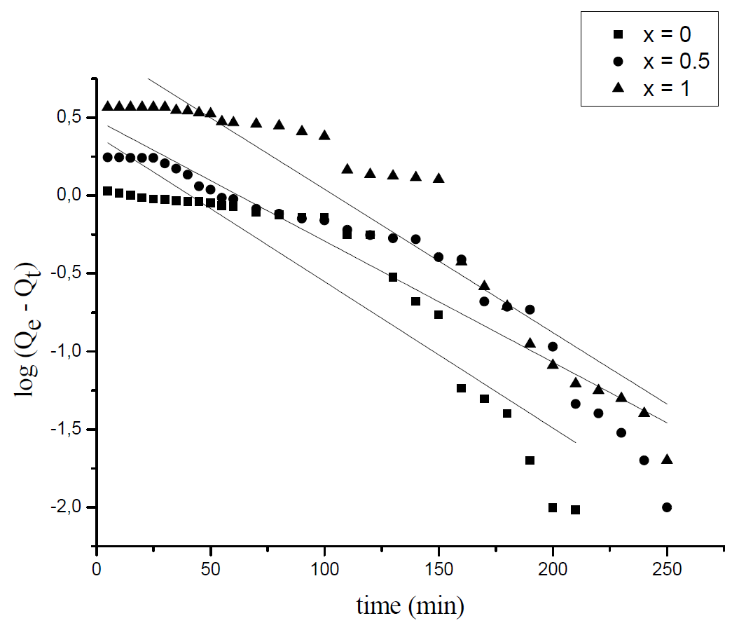 | Figure 8. The plots of log (Qe-Qt) versus time relative to MB adsorption onto Li3Fe1-xCrx(MoO4)3 |
|
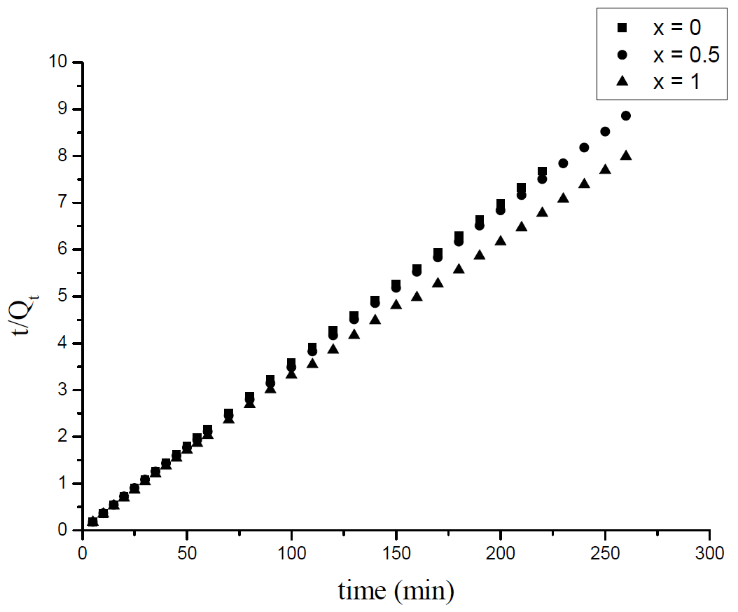 | Figure 9. The second order model fits to the experimental data for the adsorption of the MB on Li3Fe1-xCrx(MoO4)3 |
|
3.4. Adsorption Isotherms
- Adsorption isotherm describes the relationship between the adsorbed content of one substance and its amount in the equilibrium solution at fixed temperature. It facilitates the description of the interaction between the adsorbate and the adsorbent. Adsorption isotherm represents the amount of the adsorbate bounded to the surface (Qe) as a function of the material present in the solution (Ce). Fig.10 reproduces the adsorption isotherms relatives to the molybdates Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1).
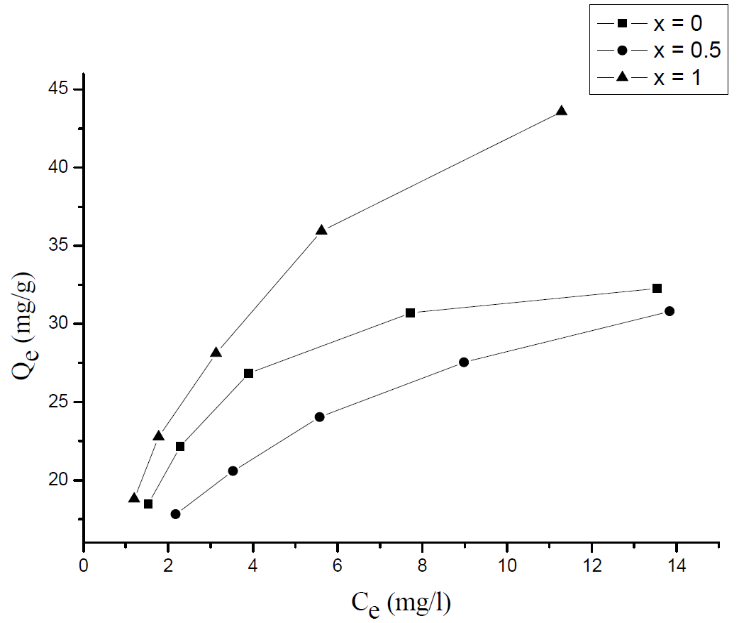 | Figure 10. Adsorption isotherm of MB onto Li3Fe1-xCrx(MoO4)3 |
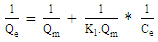 | (6) |
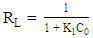 | (7) |
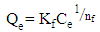 | (8) |
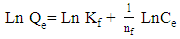 | (9) |
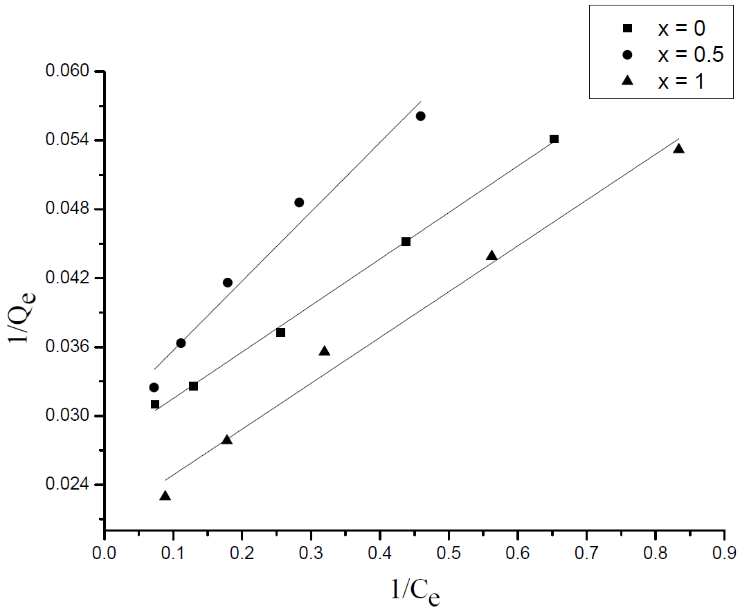 | Figure 11. Langmuir isotherm of the MB adsorption onto Li3Fe1-xCrx(MoO4)3 |
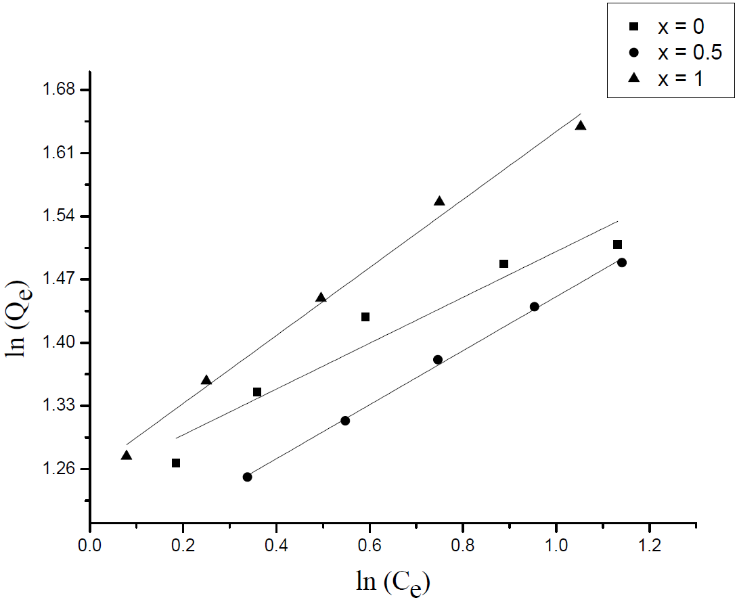 | Figure 12. Freundlich isotherm of the MB adsorption onto Li3Fe1-xCrx(MoO4)3 |
|
4. Conclusions
- The molybdates Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) have been successfully synthesized by the soft combustion method and proved to belong to the Lyonsite type structure. The adsorption studies showed that these materials can interact with the MB dyes. The results showed that the MB adsorption depended on pH of solution, temperature, adsorbent amount and contact time. The adsorption was found to be favorable with the increasing of pH of solution and it was indicated that the adsorption is more favorable at high temperature. The adsorption of MB onto Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) was best described by the pseudo-second order model. The thermodynamic parameters of the adsorption showed that the adsorption of MB onto Li3Fe1-xCrx(MoO4)3 (x = 0, 0.5 and 1) is an endothermic process and the interaction between MB and the molybdate adsorbents are physical in nature. The maximum value of the adsorption capacity calculated according to the Langmuir model for the composition (x=1) is 48.07 mg/g. It is also shown that the adsorption is very fast and the substitution of iron by the chromium has a positive effect on the adsorption.
ACKNOWLEDGEMENTS
- The authors acknowledge the Institute for Research in Solar Energy and New Energies (IRESEN) for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML