-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(2): 29-35
doi:10.5923/j.chemistry.20160602.02

Transition Metal Complexes of New N-Amino Quinolone Derivative; Synthesis, Characterization, Thermal Study and Antimicrobial Properties
Khalil K. Abid , Redha H. Al – Bayati , Ali A. Faeq
Chemistry Department, College of Science, University of Al – Mustanseriyah, Baghdad, Iraq
Correspondence to: Khalil K. Abid , Chemistry Department, College of Science, University of Al – Mustanseriyah, Baghdad, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

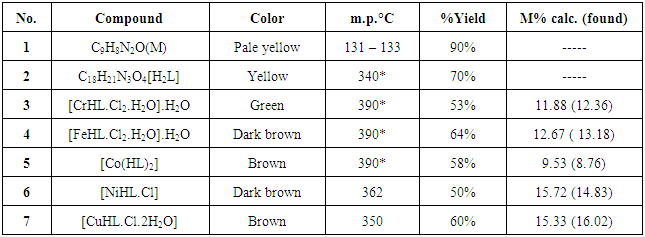
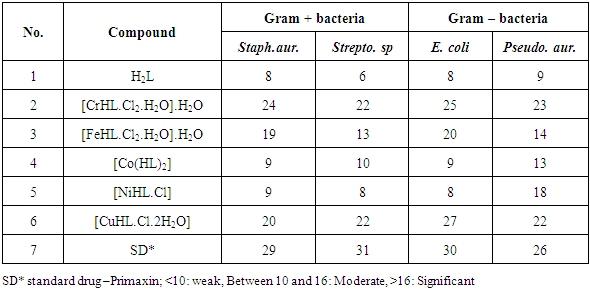
Schiff base ligand (H2L) was synthesized by the condensation reaction of 2,4 – dihydroxy benzaldehyde and N – aminoquinolone- 2 - one in 1:1 molar ratio. The ligand which exhibits ONO donor set and transition metal complexes of CrIII, FeIII, CoII, NiII and CuII ions were synthesized and characterized by 1HNMR, fourier transform infrared (FT-IR), UV – vis, elemental analysis, thermo gravimetric analysis (TGA), magnetic susceptibility and molar conductivity measurements. The ligand and metal complexes were evaluated for their anti bacterial activity using four strains of bacteria (two gram + and two gram -). The CrIII and CuII complexes showed higher activity close to the control against all the tested organisms.
Keywords: Schiff base, Transition metals, Coumarin, N – aminoquinolone - 2 - one
Cite this paper: Khalil K. Abid , Redha H. Al – Bayati , Ali A. Faeq , Transition Metal Complexes of New N-Amino Quinolone Derivative; Synthesis, Characterization, Thermal Study and Antimicrobial Properties, American Journal of Chemistry, Vol. 6 No. 2, 2016, pp. 29-35. doi: 10.5923/j.chemistry.20160602.02.
1. Introduction
- Quinolone is one of the most popular N-heteroaromatic compounds incorporated into the structures of many pharmaceuticals [1]. 2-Quinolones which are isosteric with coumarins and isomeric to 4-quinolones could become the probable potential candidate for antibacterial activity [2]. Many substituted quinoline-2-one derivatives have recently craned great interest in chemotherapy as antitumor drugs and a number of quinolones are excellent reservoir of bioactive substances [3, 4]. Recent reports show 4-hydroxy-2- quinolone derivatives are selective glycine site antagonists related to several central nervous system disorders including schizophrenia, Parkinson’s and Alzheimer’s diseases [5]. 2-Quinolones are also valuable intermediates in organic synthesis, since they are easily converted into 2-chloro and then 2-amino-quinoline derivatives [6].The preparation of ligands containing a variety of donor groups is increasing continuously and it is multiplied many fold when the ligand have biological importance [7, 8]. It has been reported that some transition metal complexes with quinolone derivatives exhibit excellent DNA-binding and biological activities [9, 10]. The mechanisms of cytotoxic action of a large number of different coordination compounds have been discussed in relation to the development of new antitumor agents and this lead to a wide range of medicinal applications of metal complexes of quinolones [11]. By considering the above facts and their increasing importance in pharmaceutical and biological field, it was considered of interest to synthesize some new chemical entities incorporating the two active pharmacophores in a single molecular frame work and to evaluate their biological activities. Only few researchers reported the synthesis and antimicrobial studies of metal complexes with Schiff bases derivative from N-amino Quinolone [12]. The present research report the preparations, characterization of new 2-quinolone derivative and complexes with Cr (III), Fe(III), Co(II), Ni(II) and Cu(II) ions. The synthesized compounds were evaluated for their antimicrobial activities against four strains of bacteria (two gram + and two gram -) and compared with a standard drug.
2. Materials and Methods
- All chemicals were analytical grade and used without any modification. 1HNMR spectra were obtained using a Bruker 300 MHz and d6 –DMSO as a solvent at University of Al Albait, Amman, Jordan. FT-IR spectra were recorded on a Shimadzu spectrophotometer. Elemental analysis for the ligand and metal complexes were carried out using CHNO—elemental analyzer and atomic absorption using Schimadzu model 6809. Electronic spectra using Varian UV–visible spectrophotometer, Molar conductivity of the complexes were measured in DMSO as a solvent in 0.001M solutions using a CON 510 bench conductivity meter with 2-ring stainless steel conductivity electrode (cell constant, K = 1.0), Magnetic susceptibility measurements were carried out using of Curie balance at the Department of Chemistry while Bacterial strains and Mueller Hinton agar were supplied from Department of Biology, College of Science, Mustanseriyah University. Thermal stability (weight changes) of the samples was recorded by Mettler Toledo TGA851 in the temperature up to 600°C at College of Education of Pure Science Ibn-Alhaithum, University of Baghdad, Iraq. Synthesis of 1-amino-2-quinolone (M)This ligand (M) was prepared according to literature [11]. Coumarin (1.46g, 0.01 mol) in ethanol (20 mL) and excess of hydrazine hydrate (98%) (5g, 0.1 mol) were stirred under reflux for 12 h., reduce the volume by evaporation, cooled and the separated solid product was filtered and washed with cold ethanol and dried. Recrystallization from ethanol to give pale yellow crystals with yields 90%, m.p. 131 – 133°C. Elemental analysis for C9H8N2O, M.wt. 160; calculated (%) C 67.50, H 5.00 , N 17.50, O 10.00; found (%) C 67.34, H 5.32, N 17.45, O 9.97. Syntheses of the ligand H2L 2,4 – dihydroxybenzaldehyde (1.12 g, 10 mmol) dissolved in ethanol (20ml) was added to M (1.60 g, 10 mmol) in ethanol (20ml). Few drops of glacial acetic acid was added as catalyst, and then refluxed for 7hrs (Scheme 1). cooled and the formed ligand was collected and recrystallized from ethanol to afford a yellow solid crystals with yield 70%, m.p.278 – 280°C. Elemental analysis for C16H12N2O3, M.wt. 280; calculated (%) C 68.57, H 4.28, N 10.00, O 17.15; found (%) C 69.09, H 5.10, N 9.89, O 17.87.
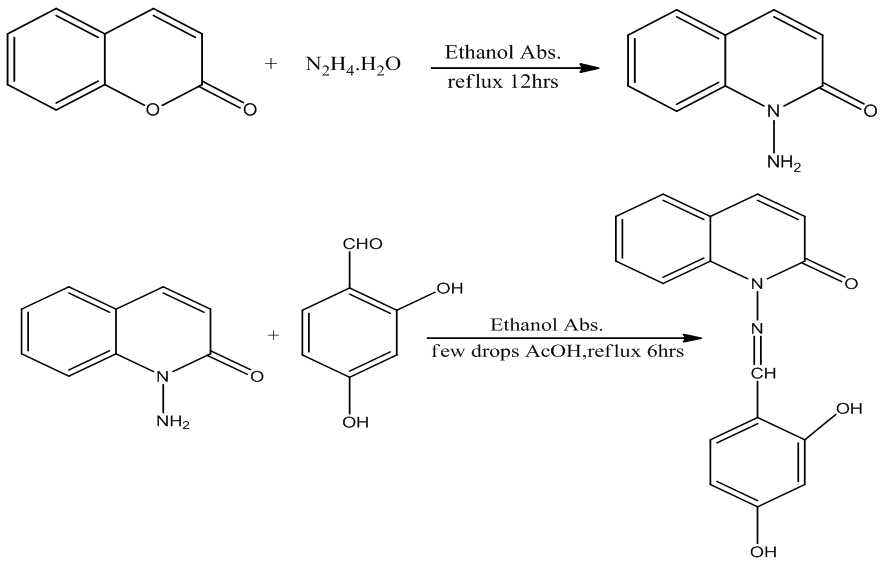 | Scheme 1. Synthesis of intermediate M and the ligand (H2L) |
|
3. Results and Discussion
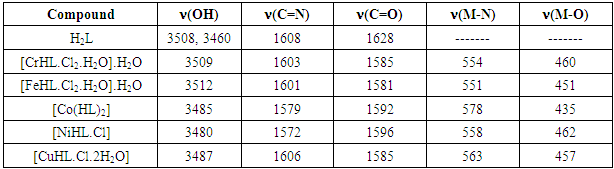
- FT – IR spectra: The spectra were recorded in the region 4000–400 cm−1 using KBr disc. The ligand (H2L) shows two absorption band at 3508 and 3460cm−1 attributed to υ (OH1) and (OH2) groups respectively. Strong band located at 1627 cm-1 is assignable to lacton υ (C=0) of coumarin. The other bands at 1608 cm-1 corresponding to imine (C=N) stretching frequency, while the aromatic ring gave two bands at 3205 and 1450 cm-1 attributed to (υ =CH) and (υ C=C), and the band assigned to aliphatic υ (C–H) appeared at 2986 – 2858 cm−1 [13]. In metal complexes, the disappearance of phenolic υ (OH1) was observed with low shift (5 – 45cm-1) for the position of the amide group (C=N), and lactone group (C=O) bands; this is indicative of formation of coordination bonds with each group. Ligand coordination to the metal centre is supported by the appearance of another two bands at approximately 551– 578 and 435 - 462 cm-1 corresponding to υ (M–N) and υ (M–O) respectively [14, 15] (Table 2). A broad band at approximately 3350 cm-1 and two other weak bands at approximately 750 and 700 cm-1 for CrIII, FeIII and CuII complexes indicate the presence of coordinated water molecule [16].
|
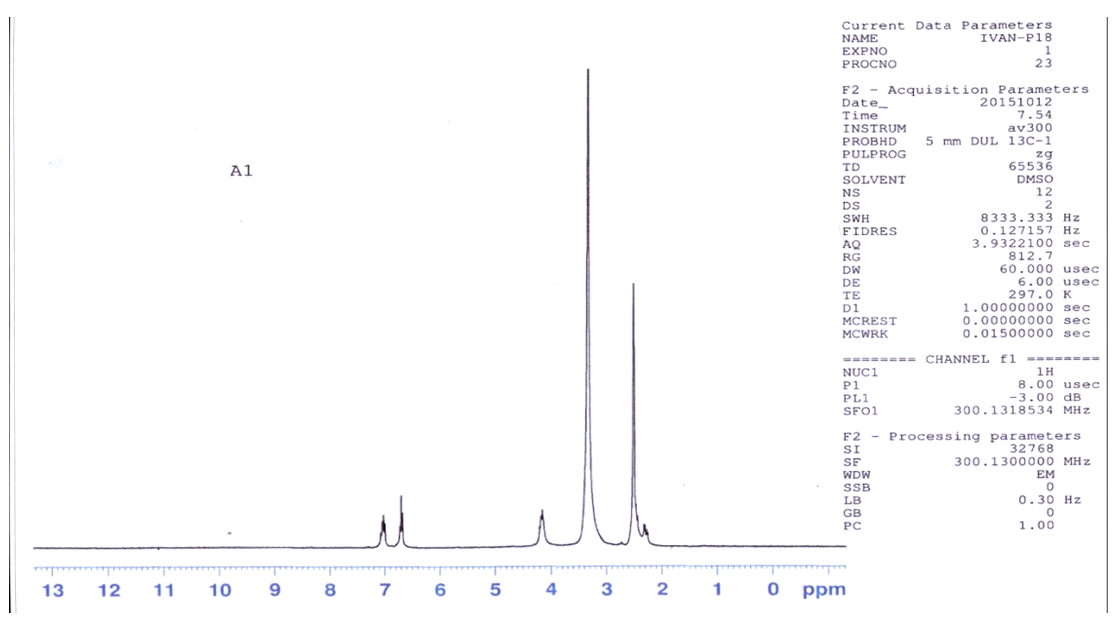 | Figure 1. 1HNMR spectrum of M |
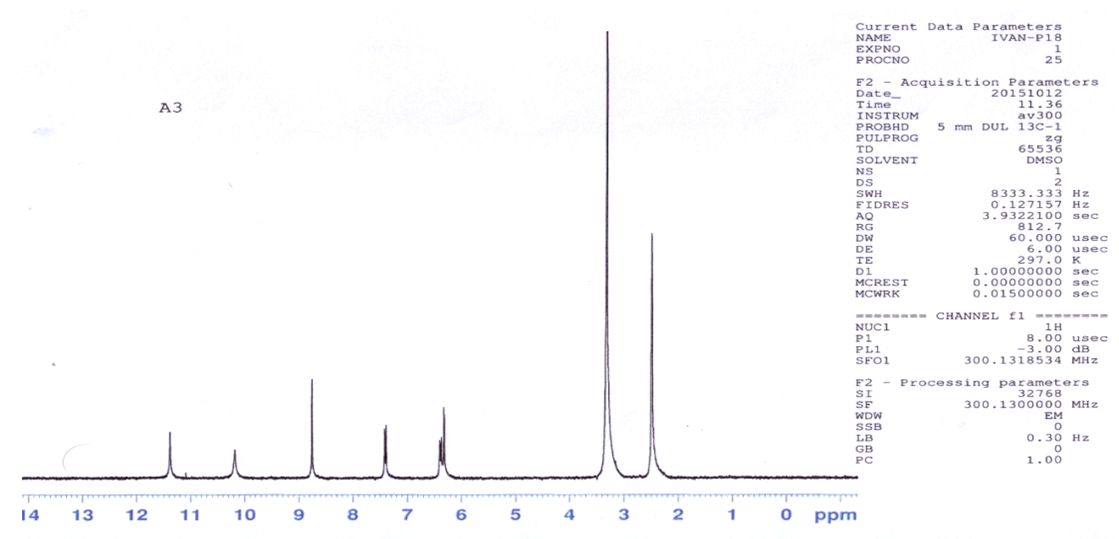 | Figure 2. 1HNMR spectrum of the ligand H2L |
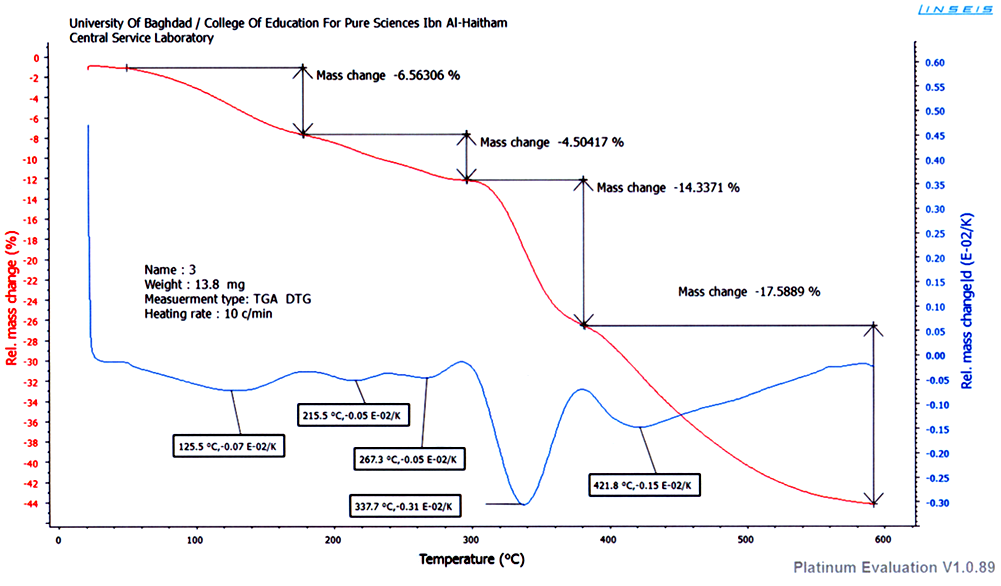 | Figure 3. TGA of CrIII complex |
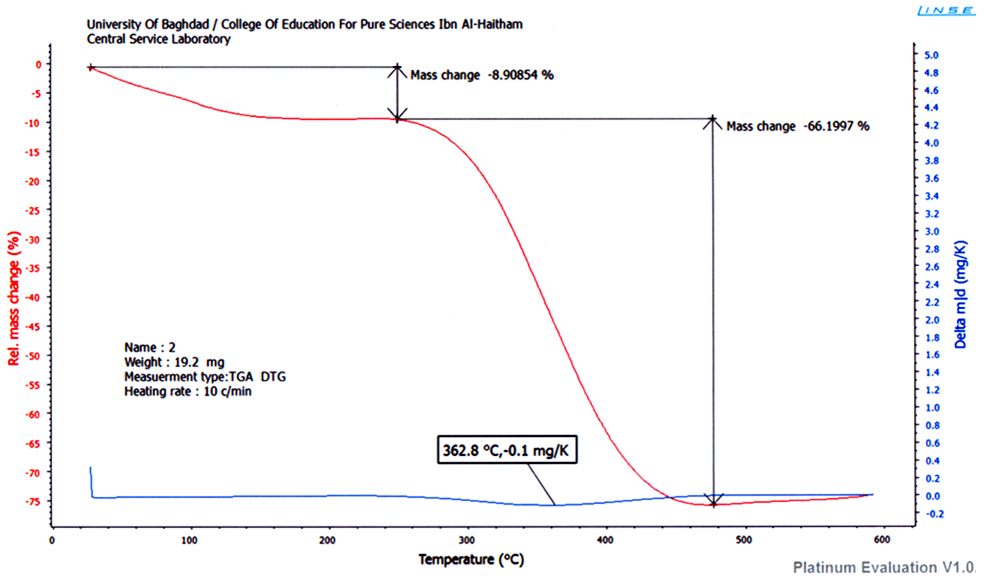 | Figure 4. TGA of CoII complex |
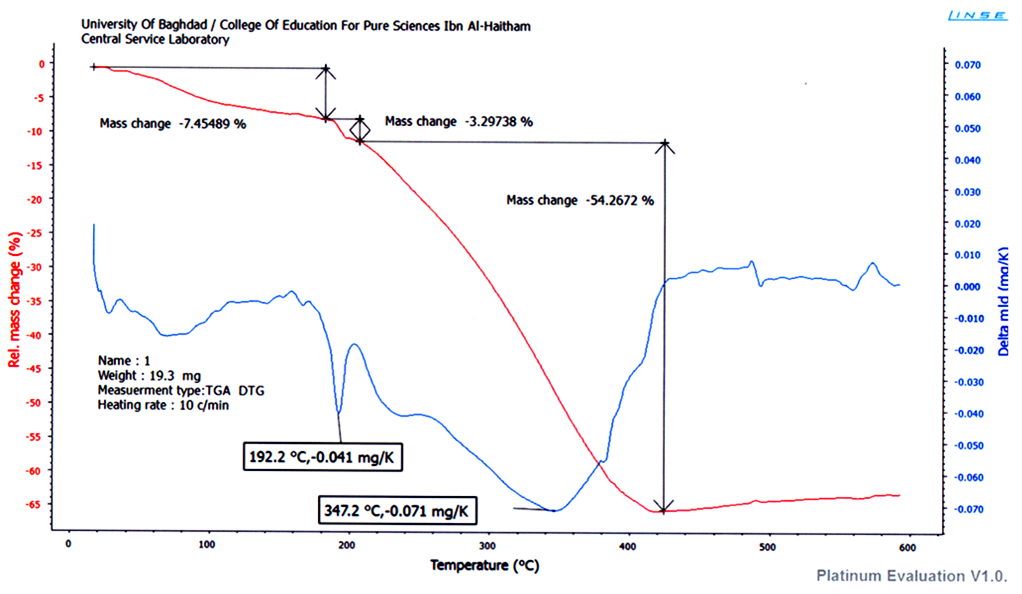 | Figure 5. TGA of CuII complex |
|
4. Conclusions
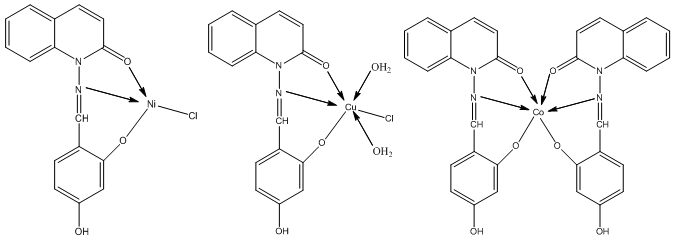
- The condensation reaction of 2,4 – dihydroxybenzaldehyde and N – aminoquinolone- 2 - one affords a new tridentate Schiff base ligand. The CrIII and CuII complexes were found to be the most active against all the tested organisms with MIC close to the standard drug control. The analytical data of the transition metal complexes of the synthesized ligand confirmed a molar ratio (1:1) M:L except CoII complex (1:2) as shown below.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML