Adegbe A. A., Larayetan R. A., Omojuwa T. J.
Department of Chemistry, Kogi State University, Anyigba, Nigeria
Correspondence to: Larayetan R. A., Department of Chemistry, Kogi State University, Anyigba, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The fixed oil from M. Oleifera seed oil was analyzed by a combination of GC and GCMS. Twenty four (24) constituents amounting to 96.81% of the total oil were identified. The major constituents were Oleic acid (22.51%), Palmitic acid (10.64%), 9-octadecenol (12.76%) and phenylbut-3-yne (5.79%). The fixed oil of M. Oleifera seed is rich in fatty acids (44.93%) followed by hydrocarbons (32.95%), others are aldehyde (12.76%) esters (3.55%) and oxygenated hydrocarbon (2.62%). The oil was found to contain moderate level of unsaturated fatty acids, mainly Oleic acid (22.51%) and Erucic acid (1.98%). The dominant saturated acids were Palmitic (10.64%), Stearic acid (6.07%), Arachidic acid (2.21%) and Docosanoic (Behenic acid) (1.03%). The physio-chemical analyses and proximate composition of the n-hexane extract of M. Oleifera seed oil were also determined using standard analytical methods. Results showed that the specific gravity and refractive index were 0.9050 & 1.456. The recorded acid value, iodine value, Saponification number, free fatty acids and peroxide values were in the order 6.73mgKOHg-1, 68.65g100g-1, 180.92mgKOHg-1, 4.21mgKOHg-1, 2.60 meqKg-1. The percentage yield of the oil was 38.0% and the color of the oil was cream yellow. The following fatty acids were identified from the GCMS analysis. Myristic acid, Oleic acid, Palmitic acid, Stearic acid, Erucic acid, Arachidic acid and Docosanoic acid (Behenic acid). The result of the proximate composition showed that the seed contain 10.50% moisture, 39.57% crude protein, 5.00% ash, 5.00% crude fiber, 32.50% fat and 7.44% carbohydrate. The seed oil of M. Oleifera showed good physio-chemical properties and could be utilized successfully as a source of edible oil for human consumption and for industrial applications.
Keywords:
Moringa Oleifera, Oil yield, Soxhlet extraction, GCMS, Physiochemical and proximate composition
Cite this paper: Adegbe A. A., Larayetan R. A., Omojuwa T. J., Proximate Analysis, Physicochemical Properties and Chemical Constituents Characterization of Moringa Oleifera (Moringaceae) Seed Oil Using GC-MS Analysis, American Journal of Chemistry, Vol. 6 No. 2, 2016, pp. 23-28. doi: 10.5923/j.chemistry.20160602.01.
1. Introduction
Moringa Oleifeira Lam belongs to an Onogeneric family of trees and shrubs Moringaceae. A single genus with known specie, M. Oleifera is the most widely known and utilized of these. [1]. It is a fast growing, aesthetically pleasing small tree; it can grow up to four (4) meters and can bear fruit within the same first year [2].Moringa Oleifera is referred to as “Moringa”, it is considered one of the world’s most useful trees. Almost every part of moringa tree can be used for food or other beneficial applications. [3]. It is known and called by different names among different people of the world, among the Yoruba people of south west Nigeria, it is called “Ewe Ile”, among the south eastern Igbo people, it is called “Okwe Oyibo”, “Gawara”, among the Fulani’s, “Zogale” among the Hausa’s “Nugyekai”, in Canada, Muringai in Tamil, Mashnga Sanga” in Malayan. In English language, M.Oleifera is also called the “Miracle tree”, “Mother’s best friend”, “Never Die” and “Benzolive tree” [4].The seeds of this wonder tree have a semi-permeable seed hull and are round in shape. Descriptively, the hull has three white wings that run from top-bottom at 120 degree intervals, the seed production is between 15,000 and 25,000 seed per year for each tree. The average weight per seed is 0.3g [5]. The dry seeds can be grounded into powder and used for seasoning sauces.The root from the young plant can also be dried and grounded for use as a hot seasoning base with a flavor similar to that of the horseradish. This is why Moringa tree has been given the name “Horseradish tree” [6]. A tasty hot sauce from the roots can also be prepared by cooking them in vinegar [6].The oil content of the de-hulled seed (Kernel) is approximately 42%, the oil is brilliant yellow. It is used as a lubricant for fine machinery such as time pieces because it has little tendency for it to deteriorate and become rancid and sticky [7]. It is also useful as vegetable cooking oil. The oil is known for its capacity to absorb and retain volatile substances and is therefore valuable in the perfumery industry for stabilizing scents; the free fatty acid content varies from 0.5-3%.The extract obtained from the leaves of Moringa in 80% ethanol contains growth enhancing principles (i.e., hormones of cytokine type). The extract can be used in the form of a foliar spray to accelerate the growth of young plants: use of the growth hormone spray will also cause the plants to be firmer and more resistant to pest and disease and will produce more and larger fruit which have a higher yield at harvest time [8].M. Oleifera seed oil is commercially known as “Ben Oil” or “Behen Oil”. The oil content is ranging from 25-40%. [9], and characterized by high amounts of oleic acid up to 75% [10] which makes it suitable for edible purpose and due to good oxidative stability.This study was carried out to evaluate the chemical constituents of n-Hexane extract of seed Oil of M.Oleifera as well as to assess the physico-chemical properties and proximate composition of Moringa- Oleifera.
2. Materials and Methods
2.1. Sample Collection and Preparation
The seeds of M. Oleifera used in this study were harvested from Oko-Arin area of Ilorin west of kwara State Nigeria. They were authenticated by Prof S.M Ayodele of Botany option (Biological Science Department) Kogi State University Anyigba Nigeria. The seeds were air-dried and grounded to powder using a mechanical grinder after the removal of the seed coat (AOCS, 2001).
2.2. Oil Extraction Procedure
The n-hexane extract was obtained by complete extraction using soxhlet extractor. 10g of the powdered seeds sample was put into a porous thimble and placed in a soxhlet extractor, using 210 ml of n-Hexane (with boiling point of about 40-60°C.) as extracting solvent for six (6) hours repeatedly until the required quantity was obtained. The oil was obtained after evaporation using a water bath at 70°C to remove the excess solvent from the extracted oil. The oil was kept in the refrigerator without further treatment until needed for further analysis.
2.3. Proximate Analysis
Proximate analysis was carried out according to the procedure of Association of Official Analytical Chemist (AOAC, 1990) to determine the moisture content, crude fiber, protein, crude fat, and carbohydrate components of the seed oil.
2.4. Percentage (%) Yield
The oil was recovered by complete evaporation of the solvent on a heating mantle, the recovered oil was transferred to a beaker and the beaker was then placed over water bath for complete evaporation of the solvent for about two hours. The volume of the oil was recovered and expressed as oil content %.
2.5. Physico-Chemical Properties Determination
The Physico-Chemical properties: Saponification value, peroxide number, iodine value, acid value, free fatty acid content, refractive index, oil yield and specific gravity were determined according to standard analytical methods recommended by (AOAC 2006).
2.6. GC and GC-MS Analysis
The GC and GC-MS analysis of the seed oil of M.Oleifera was performed using a multi dimensional gas chromatography coupled with gas chromatography-mass spectrophotometer. (Shimadzu Japan) equipped with non-polar and polar double capillary columns (25.0m×0.25µm i.d., 0.25µm df). High purity helium was used as the carrier gas at a constant flow rate of 0.99ml/min. 1 µl sample was injected (split ratio 100:1) into GC and GCMS using AOC-2Oi; auto injector for analysis. The initial temperature was set at 60°C, heated at a rate of 3°C/min to 280°C and held isothermally for 6minutes. Ion source temperature was set to 200°C while the interface was set at 250°C, solvent cut time was 3 minutes. Electron impact (EI) ionization mode was 70ev and the linear velocity of the column was 36.8cm/sec.The identification of the various components was based on comparison of their mass spectra with those of Nist Library mass Spectra data base and mass spectra from Literature.
3. Result and Discussion
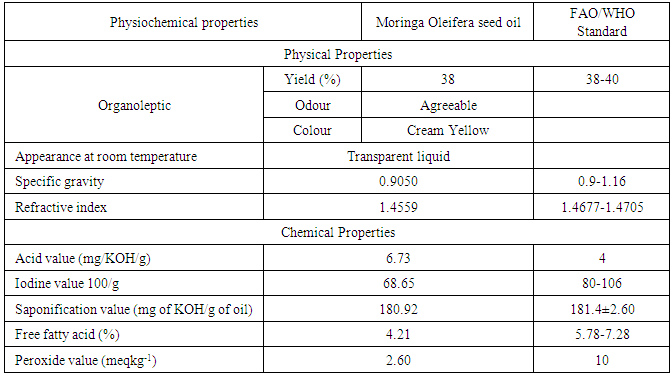
Data in table 1 represent the physio-chemical properties of M. Oleifera seed oil.Table 1. Physical and chemical properties of M. Oleifera seed oil
 |
| |
|
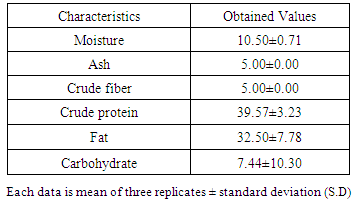
The oil extracted from M. Oleifera seed has an agreeable odour and the color is cream-yellow, the percentage oil yield is 38%. This percentage yield was higher’ than that reported by [11], where the seed oil yield of M. Oleifera was 34.50. The 38% yield of M. Oleifera was consistent with that of Literature [9]. The specific gravity of M. Oleifera seed oil was 0.9050 and this value is in agreement with the FAO/WHO (2009) [15] international standard for edible oil. The refractive index 1.4559 was in agreement with the FAO/WHO (2009) [15] international standard for edible oil. The physical properties of the oil extracted from M. Oleifera seed were in conformity with the FAO/WHO (2009) [15] standard. On the other hand, the chemical properties of the oil are shown also in table 1. An acid value of 6.73 mg/ KOHg-1, this value is higher than the acid value specified for edible oil by FAO/WHO (2009) [15] but this value was almost in agreement with Literature (5.0386 mg KOH/g) reported by [12].Iodine value is the measure of the degree of the unsaturation of the oil. Higher Iodine value indicates higher unsaturation of fats and oils. The iodine value of the oil M. Oleifera is 68.65. This is in agreement with the FAO/WHO (2009) [15] standard for edible oil, which means that most of our fatty acids are saturated. Peroxide value was 2.6 which is by far lower than the FAO/WHO (2009) [15] standard and lower than that reported by [11]. A low peroxide value as seen in our study increases the suitability of the oil for a long storage due to low level of oxidative and lipolytic activities. The Saponification value of the oil is 180.92, this value shows consistency with FAO/WHO (2009) [15] standard. On the other hand, the free fatty acid of M. Oleifera seed oil is 4.21 mgKOHg-1 which is within the range of the FAO/WHO (2009) [15] standard. High free fatty acid cause soap formation during alcoholysis process from it’s by products.Table 2. Proximate Analysis of M.Oleifera Seed Oil
 |
| |
|
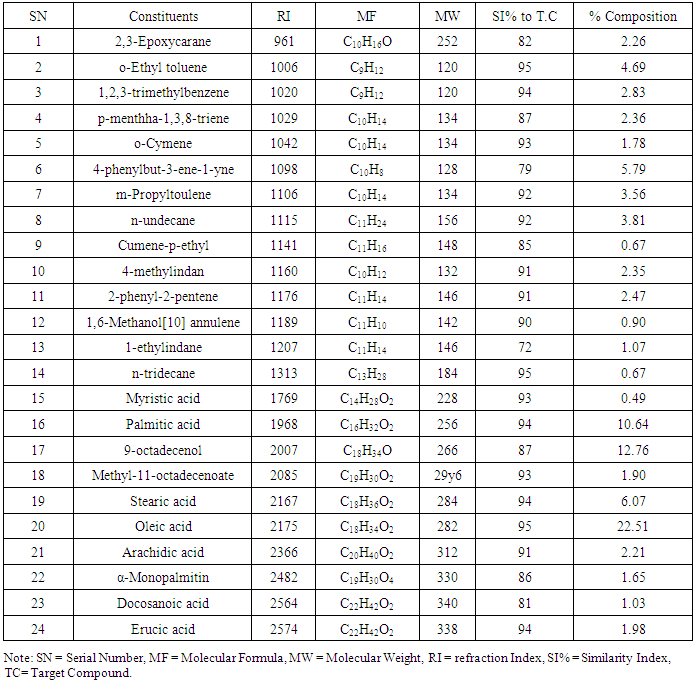
Result of the proximate composition of M.Oleifera seed oil is shown in table 2. The moisture content is 10.50%, ash content, 5.00%, crude fiber 5.00%, crude protein 39.57%, fat content 32.50% and carbohydrate (by difference) 7.44%.The observed low moisture content in M.Oleifera seed in this study serve as an indication that the activities of the micro-organisms would be reduced and thereby increases the shelf life of M.Oleifera sample. The observed moisture content value of 10.50% is higher than the value (9.40%) reported by [21]. The ash content is 5.00% which is higher than the value (3.87%) reported by [21] but in agreement with the value (5.00%) reported by [22]. Ash is an incombustible residue left after complete combustion of any substance.The crude fiber content of 5.00% obtained in our sample was higher than 2.87% reported by [21] but lower than 20.00% reported by [22] from Ebonyi, Crude Fiber content has been established to help in bowel movement. Adequate intake of dietary fiber can lower cholesterol level, risk of coronary heart diseases, constipation, hypertension, diabetes, colon and breast cancer [23, 24]. Crude protein and fat content are 39.57% and 32.50% respectively. The crude protein content is higher than 35.97% and 9.98% reported by [21, 22] while the observed fat content in this study is lower than 38.62% and 40.00% reported by [21, 22]. As reported by Pearson (1976), plant food that provide more than 12% of its calorific value from protein, is considered good source of protein. Therefore, M. Oleifera is a good source of protein. Carbohydrate content is 7.44% which is lower compared to the value 18.00 reported by [22].The chemical components of the fixed oil M. Oleifera seed oil was analyzed using multi-dimensional gas chromatography coupled with gas-chromatography-mass spectrophotometer (GC-MS). Twenty four (24) components amounting to 96.81% were identified in the seed oil. The identified components, their retention indexes and percentage composition of each component are given in the table above. The major constituents found in the fixed oil of M. Oleifera seed oil are: Oleic acid (22.51%), Palmitic acid (10.64%), Stearic acid (6.07%), 9-octadecenal (12.76%) and Phenyl but-3-1-yne (5.79%). Other noticeable constituents found in the oil were o-Ethyltoulene (4.64%), m-Propyltoulene (3.56%), 4-methylindin (2.35%), 2-phenyl-2-pentane (2.47%), p-mentha -1, 3, 8-triene (2.36%) and arachidic acid (2.21%). It is worth mentioning that the main compounds characterizing the fixed oil of M. Oleifera are qualitatively and quantitatively different. The fixed oil of M. Oleifera is rich in fatty acid (44.93%) followed by hydrocarbons (32.95%), others are aldehyde (12.76%), esters (3.55%) and Oxygenated hydrocarbons. (2.62%). The following fatty acids were identified from the GC-MS analysis: Oleic acid (22.51%) was the major component of the fixed oil. Oleic acid is a mono-saturated omega-9-fatty acid with many health’s benefits and is safe in present practices for use and concentrations in cosmetics [17]. Oleic acid prevents ulcerative colitis [25], protects cell from free radical damage [26], reduces blood pressure [27] and increases fat burning [28]. Palmitic acid is a saturated long chain fatty acid with sixteen carbon backbone. It is one of the most abundant and wide spread natural saturated acids present in plants like palm oil, palm kernel oil, M. Oleifera seed oil, in animals and animal-derived foodstuffs like cheese, milk, meat and microorganisms [28]. It is among the fatty acid that is used as concentration in cosmetics [29].Table 3. GCMS Chemical Constituents of M. Oleifera seed oil
 |
| |
|
Arachidic acid is a saturated long-chain fatty acid with 20-carbon backbone found naturally as a minor component of peanut oil, also found in M. Oleifera seed oil. Its IUPAC name is icosanoic acid C20H40O2. It is used in the industry as component of adhesive, sealant, lubricants, as lubricant additive or in agricultural products.Stearic acid, a saturated fatty acid having 18-carbon chain was formed in M. Oleifera seed oil. It has an IUPAC name of octadecanoic acid, stearic acid is mainly used in the production of detergent, soaps and cosmetics such as shampoos and sharing cream products. Soap is not made directly from stearic acid but indirectly by Saponification of triglycerides consisting of stearic acid esters. Surfactants, cosmetics and personal hygiene products are in fact prospects of stearic acid [18]. Myristic acid was also found as one of the constituents of M. Oleifera seed oil. It is a saturated fourteen (14) carbon fatty acid found naturally in palm oil, coconut oil and butter fat. Myristic acid is used as a flavoring agent in food [29] also used as emulsifiers, emollient and lubricants in variety of cosmetics, creams, cake, soaps & pastes [30, 31]. It is an important fatty acid which the body uses to stabilize many different proteins, including protein in the immune system.Erucic acid also known as (Z)-docos-13-enoic acid (IUPAC) was also present in M. Oleifera seed oil. It is a monounsaturated omega-9-fatty acid with twenty-two carbon atoms. It is a major constituent of certain oils, such as rapeseed oil. This oil is linked to cardiac muscle damage.Docosanoic acid (Behenic acid) is a major component of Ben oil which is extracted from the seed of M. Oleifera tree [19] and found in the seeds of M. Oleifera sample of this present study. It is used to give hair conditioners and moisturizers their smoothing properties. Also uses as antifoam in the manufacturing of detergents [20].
4. Conclusions
This oil could be utilized successfully as a source of edible oil for human consumption. The physio-chemical parameters of the oil are comparable to those of other edible oil, therefore flour from M.Oleifera seeds could be employed in the fortification of other food materials. The result also showed that the properties of M.Oleifera oil in Nigeria could be employed for edible and cosmetics application. The seed oil exhibited good physio-chemical properties and could be useful for industrial applications.
References
| [1] | Morton JF (1991). The Horseradish tree, Moringa pterygosperma. A boom to arid lands. Economic Botany, 45:318-333. |
| [2] | Olivera JT, Salvela SB, Vasconcelos KM, Cavada BS, Morira RA (1997). Compositional and Nutritional Attributes of Seeds from the Multipurpose Tree. Moringa Oleifera. Lamarck. Journal of Science and Food Agric. 79(6):815-820. |
| [3] | Quattrocchi and Umberto (2000). CRC World Dictionary of Plants Names: Common Names, Scientific Names, Eponyms, Synonyms and Etymology. 3. CRC Press. P. 1731. |
| [4] | Ramahandran CA, Peter KV, Gopalakrishnan PK. (1980). Drumstick (M.Oleifera): a multipurpose Indian Vegetable. Economy Botany. 34(3): 276-2. |
| [5] | a) Makkar HPS and Becker K (1997). Nutrients and Antiquality factors in different morphological parts of Moringa Oleifera tree. Journal of Agricultural and Science, Cambridge 128, 312-322. b) Morton JF (1991). The Horseradish tree, Moringa pterygosperma. A boom to Arid lands. Economic Botany,45, 318-333. |
| [6] | Deleveau P and Boiteau P. (1980). Huiles a Interet Pharmacologogue, Cosmetologigue et dietaue iv. Huiles de Moringa Oleifera Lamk. et.de.M.Droulardii Jumelle. Plantes medicinales et Phytotherapie. 14,29-33. |
| [7] | Ferrao AMB & Ferrao JEM (1970). Acidos gordos em oleo de Moringueiro. Anronomia Angolana 6, 3-16. |
| [8] | Makkar HPS and Becker K (1996). Nutritional value and antinutritional components of whole and ethanol extract from Moringa Oleifera leaves. Animal Feed Scineco and Technology 63: 211-228. |
| [9] | Lalas S and Tsaksins J. (2002). Characterization of Moringa Oleifera seed oil variety. “Periyakulam 1”. J. Food Comp. and Anal. 15: 65-77 |
| [10] | Anwar F, Zafas SN and Rashid U. (2006): Characterization of Moringa Oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. GRASAS Y. ACEITES, 57(2):160-168. |
| [11] | AOCS (2001). Official methods and recommended practices of the American Oil Chemists Society. 6 th Edn., 2001, AOCS Press, Champaign. |
| [12] | AOAC (1990)j. Official Method of Analysis. 15 th Edn., Association of Official Analytical Chemists (AOAC), Washington, DC., USA. |
| [13] | AOAC (2006). Official Method of Analysis of AOAC International. 18 th Edn., Gaithersburg, USA. |
| [14] | Arafat MG. (2013). Physicochemical properties of oil produced from Moringa Oleifera, Jatropha curcus and Carthamus tintorius L seeds. Int. J of Advanced Research. Vol 1(4): 181-187. |
| [15] | FAO/WHO (2009). Report on the 21st session of the Codex Alimentarius Committee on fats and oils. Kola Kinabalu, Malaysia. |
| [16] | Farooq A and Umar R (2007). Physicochemical characteristics of M.Oleifera seed and seed oil from a wild province of Pakistan. J.Bot. 39(5):1443-1453. |
| [17] | Liebert MA. (1987). Final Report on the Safety Assessment of Oleic acid, Laurie acid, Palmitic acid, Myristic acid and Stearic acid. Journal of American College of Toxicology. 6(3): 321-402. |
| [18] | Gunstone FD (2014). The Chemistry of Oils and Fats, sources, composition, properties and uses. Blackwell Publishing Ltd, UK. |
| [19] | Warra AA. (2014). Cosmetic Potential of Oil Extracts from Seeds and Nuts Commonly Found in Nigeria. Ahmadu Bello University Press Limited, Zaria, Nigeria. Pp63-64. |
| [20] | Bulus A. (2001). Behenic Acid Notification. Flamm Associates. Vero Beach. Pp:3-4. |
| [21] | Peter TO, Philip CAN (2014). Proximate Analysis and Chemical Composition of Raw and Defatted Moringa Oleifera Kennel. Advances in Life and Tech. J. Vol 24. |
| [22] | Aja PM, Ibiam UA, Uraku AJ, Orji OU, Offor CE and Nwali BU (2013). Comparative, Proximate and Mineral Composition of Moringa Oleifera Leaf and Seed. Global Advanced Research Journal of Agricultural Science. Vol 2 (5). Pp137-141. |
| [23] | Ishida HH, Suzono N, Sugiyama S, Innami T, Tadokoro and Maekawa A. (2000). Nutritive Evaluation on chemical components of leaves, stalks, stems of sweet potatoes. (Ipomoea batatas poir). Food Chem; 68: 359-367. |
| [24] | Rao CV, Newmark HL and Reddy BS. (1988). Chemo-preventive effect of Squalene on colon cancer. Carcinogenesis, 19:287-290. |
| [25] | De Silver PS, Luben R, Shrestha SS, Khaw KT, Hart AR. (2014). Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: A prospective cohort study using 7-days food diaries. Eur. J. Gastroenterol Hepatol. 26 (1): 11-8 [PubMed] |
| [26] | Haug A, Hestmark AT and Harstad OM. (2007). Bovine milk in human nutrition-a review. Lipid Health and Disease. 6:25. [PubMed]. |
| [27] | Ruiz-Gutirrez V, Munana FJ, Guerrero A, Cert AM, Villar J. (1996). Plasma Lipids erythrocytes membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic from two different sources. J. Hypertens. 14: 1483-1490. [PubMed]. |
| [28] | Lim JH, Gerhart-Hines Z, Doming JE, Lee Y, Kim S, Tabata M, Xiang YK, Puigserver P. (2013). Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α-complex-J. Biol Chem. 288:7117-26. [PubMed]. |
| [29] | Fassett DW and Irish DD. (1963). Industrial Hygiene and Toxicology. 2nd Edn. Toxicology. New York: Interscience Publishers. Vol 2. |
| [30] | Osol A (ed). Remington’s Pharmaceutical Sciences, 16th Edn.Easton, PA: Mack Publ. Co. |
| [31] | Balsam MSD and Sagarin E. (1972). Cosmetics: Science and Technology, 2nd Edn. New York: John Wiley and Sons, Vol 1, 2, 3. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

