| [1] | J. E. Gowan and T. S. Wheeler, Name Index of Organic Reaction, London, U.K.: Longmans, 1960, pp. 10-11. |
| [2] | Baeyer, A. and Drewsen, V., 1882, Darstellung von Indigblau aus Orthonitrobenzaldehyd, Chem. Ber.,15(2), 2856-2864. DOI: 10.1002/cber.188201502274 J. Chem. Soc., Abstr., 44, 341-342, 1883. |
| [3] | Baeyer, A. and Drewsen, V., 1883, Einwirkung von Orthonitrobenzaldehyd auf Aldehyd, Chem. Ber., 16(2), 2205-2208. DOI: 10.1002/cber.188301602131 J. Chem. Soc., Abstr., 46, 58-59, 1884. |
| [4] | L. Gattermann, Laboratory Methods of Organic Chemistry, New York, USA: Macmillan, 1957, pp.371-372. |
| [5] | Tanasescu, I. and Georgescu, A., 1932, Sur le mécanisme de formation de l’indigo, dans la synthèse de v. Baeyer, Bull. Soc. Chim. France, 51, 234-240. |
| [6] | N. Ono, The Nitro Group in Organic Synthesis, Wiley Series in Organic Nitro Chemistry, Germany: Wiley-VCH, 2001, pp.30-69. ISBN: 978-0-471-31611-4. |
| [7] | P. Karrer, Organic Chemistry, 3rd. ed., Amsterdam, Holland: Elsevier, 1947, p. 557. |
| [8] | Ranganathan, S., 1996, The Story of Indigo, Resonance-Journal of Science Education (Bangalore, India), 1(8), pp. 22-26. |
| [9] | Z. Wang, Ed., Comprehensive Organic Name Reactions and Reagents, Part. One, 32. Baeyer-Drewson Reaction: Wiley, Online: 15 Sept. 2010. DOI:10.1002/9780470638859.conrr032. |
| [10] | Baeyer-Drewson indigo synthesis, https://en.wikipedia.org/wiki/Baeyer-Drewson_indigo_synthesis Accessed: Dec. 15, 2015. |
| [11] | A. Parikh, H. Parikh and K. Parikh, Name Reactions in Organic Synthesis, India: Foundation Books, 2006, Chapter 4, Baeyer-Drewson Indoxyl Synthesis, pp. 464-466. ISBN: 9788175963788 |
| [12] | I. T. Millar, A Shorter Sidgwick’s Organic Chemistry of Nitrogen, Oxford, UK: Clarendon, 1969, p. 322. |
| [13] | V. Migrdichian, Organic Synthesis, New York, USA: Reinhold, 1960, Vol. 2, pp. 1631-1632. |
| [14] | G. Hilgetag and A. Martini, Eds., Weygand-Hilgetag Preparative Organic Chemistry, New York, USA: Wiley, 1972, pp. 447-448. |
| [15] | Protocole expérimental de la synthèse de l’indigo. https://spclasserre.files.wordpress.com/2014/09/tp7-indigo.pdf Accessed: Dec. 18, 2015. |
| [16] | Lab-synthesis of Indigo. http://chemlab.truman.edu/Chemistryofartlabs/Synthesis of indigo.pdf Accessed: Dec. 19, 2015. |
| [17] | W. J. Hickinbottom, Reactions of Organic Compounds, 3rd. ed., London, UK: Longmans, 1962, p.72. |
| [18] | I. T. Harrison and Sh. Harrison, Compendium of Organic Synthetic Methods, New York, USA: Willey-Interscience, 1971, p.147. |
| [19] | Synthesis of benzaldehydes from toluene and its derivatives. http://chemistry.mdma.ch/hiveboard/chemistrydiscourse/000208702.html Accessed: Dec. 30, 2015. |
| [20] | F. Giral and C. A. Rojahn, Productos Químicos y Farmacéuticos, Vol. 2, Mexico City, Mexico: Atlante, 1956, pp. 1011-1013. |
| [21] | Popkov, A., 2005, Synthesis of 2-nitrobenzaldehyde from 2-nitrotoluene, Acta Chim. Slov., 52(4), 460-462. |
| [22] | R. C. Fuson, Advanced Organic Chemistry, New York, USA: Wiley, 1958, p. 278. |
| [23] | L. F. Fieser and M. Fieser, Reagents for Organic Synthesis, New York, USA: Wiley, 1967; Acetyl nitrate, p. 13. |
| [24] | A. V. Topchiev, Nitration of Hydrocarbons and Other Organic Compounds, London, UK: Pergamon, 1959, p. 295. |
| [25] | Sánchez-Viesca, F., Gómez, M. R. and Berros, M., 2011, Electric hindrance and precursor complexes in the regio-chemistry of some nitrations, J. Chem. Ed., 88(7), 944-946. DOI: 10.1021/ed900030s |



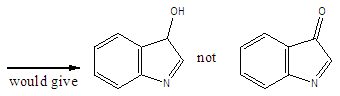
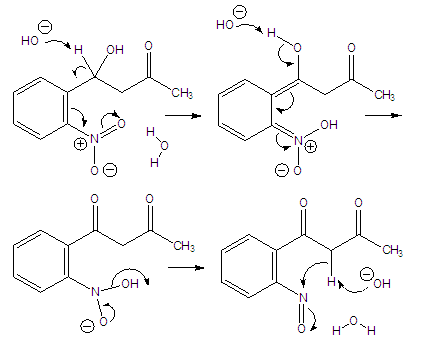
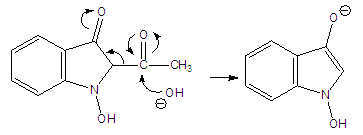
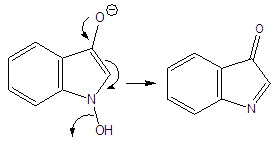
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML