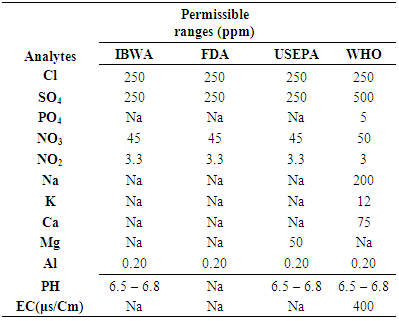
| [1] | IBWA International Bottled Water Association, Alexandria, VA 22314, 2004. |
| [2] | Natural Resources Defense Council (NRDC) New York, NY10011, March 1999. |
| [3] | U.S. Environmental Protection Agency. Providing Safe Drinking Water in America. National Public Water System Annual Compliance Report and Update on Implementation of the 1996 Safe Drinking Water Act Amendments, Executive Summary. U.S. Environmental Protection Agency, Washington, DC, September, 1996. |
| [4] | Food and Drug Administration, The FDA Bottled Water Regulations. Code of Federal Regulations, Parts 129 and 165, Title 21; Food and Drug Administration: Rockville, Maryland, 1997. |
| [5] | Soupioni, M.J.; Symeopoulos, B.D.; Papaefthymiou, H.V. Determination of trace elements in bottled water in Greece by instrumental and radiochemical neutron activation analyses. J. Radioanal. Nucl. Chem. 2006, 268(3), 441–444. |
| [6] | Batarseh, M.I. The quality of potable water types in Jordan. Environ. Monitor. Assess. 2006, 117, 235–244. |
| [7] | Momani, K.A. Chemical assessment of bottled drinking waters by IC, GC, and ICP-MS instrumentation. Sci. Technol. 2006, 34, 587– 605. |
| [8] | Raj, S.D. Bottled water: How safe is it? Water Environ. Res. 2006, 77(7), 3013–3018. |
| [9] | Saleh, M.; Ewane, E.; Jones, J.; Wilson, B. Chemical evaluation of commercial bottled drinkingwater from Egypt. J. Food Comp. Anal. 2001, 14, 127–152. |
| [10] | Lalumandier, J.A. Fluoride and bacterial content of bottled water vs. tap water. Arch. Fam. Med. 2000, 9, 246–250. |
| [11] | Armas, A.B.; Sutherland, J.P. A survey of the microbiological quality of bottled water sold in the UK and changes occurring during storage. Int. J. Food Microbiol. 1999, 48, 59–65. |
| [12] | Warburton, D.; Harrison, B.; Crawford, C.; Foster, R.; Fox, C. A further review of the microbiological quality of bottled water sold in Canada: 1992–1997 survey results. Inter. J. Food Microbiology, 1998, 39, 221–226. |
| [13] | Schindler, P.R. Enterobacteria in mineral, spring and table water. Gesundheitswesen 1994, 56, 690–693. |
| [14] | Rivera, F.; Glavan, M.; Robles, E. Bottled mineral waters polluted by protozoa in Mexico. J. Protozool. 1981, 28, 54–56. |
| [15] | Salazar, H.C.; Moura, H.; Ramos, R.T. Isolation of free-living amoebas from bottled mineral water. Rev. Saude Publica. 1982, 16, 261– 267. |
| [16] | Caroli, G.; Levre, E.; Armani, G.; Biffi-Gentili, S.; Molinari, G. Search for acid-fast bacilli in bottled mineral waters. J. Appl. Bacteriol. 1985, 58, 461–464. |
| [17] | Papapetropoulou, M.; Tsintzou, A.; Vantarakis, A. Environmental mycobacteria in bottled table waters in Greece. Can. J. Microbiol. 1997, 43, 449–502. |
| [18] | Hunter, P.R.; Burge, S.H.; Hornby, H. An assessment of the microbiological safety of bottled mineral waters. Rivista Italianad Igiena 1990, 50, 394–400. |
| [19] | Warburton, D.W. A review of the microbiological quality of bottled water sold in Canada. Part 2. The need for more stringent standards and regulations. Can. J. Microbiol. 1993, 39, 158–168. |
| [20] | Warburton, D.W.; Bowen, B.; Konkle, A. The survival and recovery of Pseudomonas aeruginosa and its effect upon salmonellae in water: methodology to test bottled water in Canada. Can. J. Microbiol. 1994, 40, 987–992. |
| [21] | Kerr, M.; Fitzgerald, M.; Sheridan, J.J.; McDowell, D.A.; Blair, I.S. Survival of Escherichia coli O157:H7 in bottled natural mineral water. J. Appl. Microbiol. 1999, 87, 833–841. |
| [22] | Standard Methods for the Examination of Water and Wastewater, 21th ed. American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF), 2005. |
| [23] | Martin, T.D.; Martin E.R. Method 200.8, Revision 5.4, U.S. Environmental Protection Agency: Washington, DC, 1996. |
| [24] | Reasoner, D.J. Heterotrophic plate count methodology in the United States. Int. J. Food Microbiol. 2004, 92, 307–315. |
| [25] | Derry, C.; Bourne, D.; Sayed, A. The relationships between the hardness of treated water and cardiovascular disease mortality in South Africa urban areas, S Africa Med. J. 1990, 77, 522–524. |
| [26] | Al-Redhaimen, K.; Abdel-Magid, H. Magnesium and other elements and cardiovascular disease. Water Air Soil Pollut. 1985, 137, 235–246. |
| [27] | Zoeteman, B.C.J. Sensory Assessment of Water Quality. In Pergamon Series on Environmental Science Volume 2. Pergamon Press, Exeter, UK.1980, 189–210. |
| [28] | Hardisson, A.; Rodriguez, M.; Burogs, A.; Diaz Flores, L.; Gutierrez, R.; Varela, H. Fluoride levels in publicly and bottled drinking water in the island of Tenerife, Span. Bull. Environ. Contam. Toxicol. 2001, 67, 163–170. |
| [29] | Guidelines for Canadian Drinking Water Quality, Supporting Documentation- Lead; Department of National Health and welfare (Canada): Ottawa, 1978. |
| [30] | Alabdulaaly, A. Nitrate concentrations in Riyadh drinking water supplies. Environ. Monit. Assess. 1997, 47, 315–324. |
| [31] | Food and Drug Administration, What Americans are drinking in 2002. Food and Drug Administration: Washington, DC (2002). |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML