-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(1): 8-11
doi:10.5923/j.chemistry.20160601.02

Synthesis and Structural Analysis of a Dioxovanadium (V) Complex Incorporating Pyridoxal-Thiosemicarbazone (PLTSC) Ligand
Sulaiman A’Shidhani1, Mohammed Al Bouromi1, Saif Al Ameri2, Said Al Ghawi2, Violeta Jevtovic2
1Department of Biological Sciences & Chemistry, (Master Program) College of Arts and Sciences, University of Nizwa, Oman
2Department of Biological Sciences & Chemistry, College of Arts and Sciences, University of Nizwa, Oman
Correspondence to: Violeta Jevtovic, Department of Biological Sciences & Chemistry, College of Arts and Sciences, University of Nizwa, Oman.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

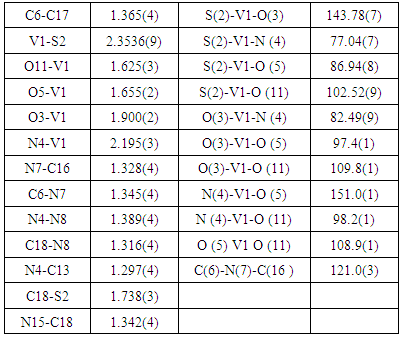
Reaction between the NH4VO3 and pyridoxal-thiosemicarbazone (PLTSC) in ammonia / methanol solution forms an orange, mononuclear [VO2 (PLTSC–H)]•2H2O complex in which vanadium is in the oxidation state +5, and pyridoxal thiosemicarbazone is coordinated in its monoanionic form. The coordination environment around vanadium can be described as an almost ideal square-pyramid.
Keywords: Pyridoxal-thiosemicarbazone, V(V) complex, Synthesis, Crystal structure
Cite this paper: Sulaiman A’Shidhani, Mohammed Al Bouromi, Saif Al Ameri, Said Al Ghawi, Violeta Jevtovic, Synthesis and Structural Analysis of a Dioxovanadium (V) Complex Incorporating Pyridoxal-Thiosemicarbazone (PLTSC) Ligand, American Journal of Chemistry, Vol. 6 No. 1, 2016, pp. 8-11. doi: 10.5923/j.chemistry.20160601.02.
Article Outline
1. Introduction
- History of research involving mixed ligand systems based on pyridoxal (Pyridoxal, 3-hydroxy-5-hydroxyrnethyl-2-methylpyridine-4-carbaldey-de), is one of the pyridoxin forms (vitamin B6) and semi-, tiosemi- and isotiosemi-carbazones is quite recent. About 30 years have passed since the first published paper from Pelizzi group [1] describing ligand (PLTSC) synthesis, as well as its first complexes preparation. Nevertheless, it was enough time to accumulate a substantial number of publications in this field. A large number of complexes incorporating PLTSC (pyridoxal - thiosemicarbazone) and PLSC (pyridoxal- semicarbazone) ligands has been reported. Complexes incorporating PLITSC (pyridoxal – S - methylisothiosemicarbazone) [2-16], were the main subject of a review [17] and a monograph [18]. With a variety of complex synthesized by various research groups, there was a good opportunity for compare different complexes which are composed of the same central metal (vanadium), but three different types of ligands (PLSC, PLTSC and PLITSC) around the metal center. Specifically, in this article we will describe the structure characterization of a vanadium complex stabilized by PLTSC (pyridoxal-thiosemicarbazone). Previously vanadium complexes with PLSC [9] and PLITSC [19] were prepared.
2. Experimental
- All commercially obtained reagent-grade chemicals wereused without further purification, except for the ligands which were prepared as mention below in the synthesis sub-section.
2.1. Synthesis of PLTSC
- Mixture of 2.03 g (10 mmol) PL·HCl, 0.91 g (10 mmol) TSC i 0.70 g (10 mmol) LiOAc was perfused with 20 cm3 MeOH and refluxed for 45 min. A yellow deposit of the ligand was isolated very fast; when heated, it was percolated and washed with MeOH. Yield: 2.42 g (82 %).
2.2. Syntheses of Complex [VO2 (PLTSC-H)]·2H2O
- Mixture of 0.20 g (0.7 mmol) PLTSC·3H2O and 0.11 g (1 mmol) NH4VO3 was perfused in a mixture of concentrated aqueous solution of ammonia (5 ml) and methanol (5 ml). The resulting mixture was refluxed in period of 1.5 h Orange solution was left at room temperature for 50 hours. The obtained crystals were filtered off and dried in vacuum. Yield: 0.24 g (88 %).
2.3. Crystal Structure Determination
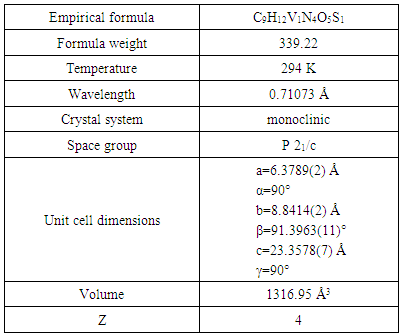
- Data for complex were collected on a Philips PW1100 diffractometer with MoKα radiation [λ = 0.7107Å]. The structure was solved using direct methods SIR92 [20] and refined using SHELXL97 [21] on F2 by full matrix least squares with anisotropic displacement parameters for all non-hydrogen atoms. Details concerning crystal data and refinement are given in Table 1. Crystallographic data have been deposited with the Cambridge Crystallographic Data Base as CCDC reference number 1438796 for the complex.
|
3. Results and Discussions
3.1. Synthesis and Structure of Complexes
- Pyridoxal-thiosemicarbazone (PLTSC, H2L), is synthesized according to formerly described procedure [18], by reaction of a warm methanol mixture of PL-hydrocloride (PL·HCl),[3-hydroxy-5-hydroxymethyl-2-methylpyridine-4-carbaldehydehydrochloride] and thiosemicarbazide (TSC·H2O) in the presence Na2CO3·10H2O (Scheme 1.)
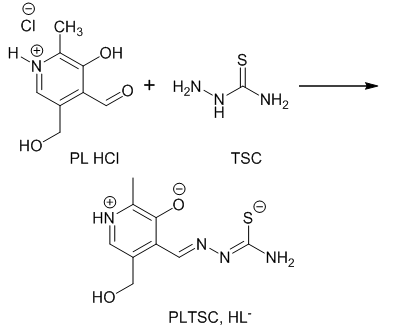 | Scheme 1. Synthesis of Pyridoxal-thiosemicarbazone (PLTSC) |
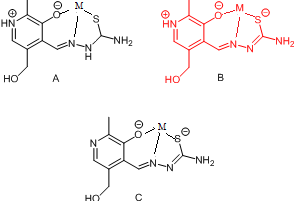 | Scheme 2. Coordination models and ligand forms: a) neutral, b) mono- and c) dianionic |
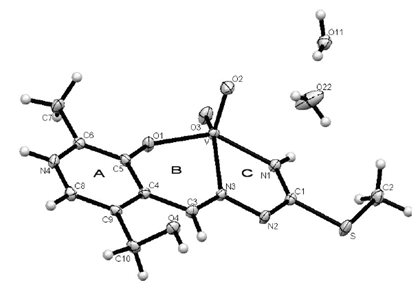
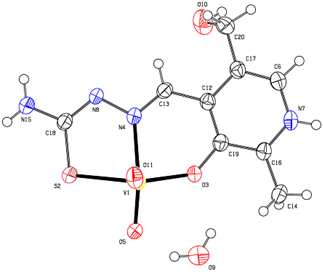 | Figure 1. The molecular structure of the complex [VO2(PLTSC-H)]·2H2O, with the atom and ring-labeling scheme |
|
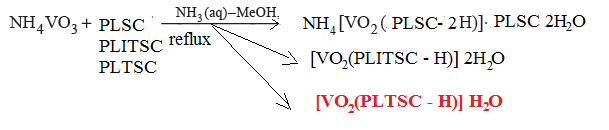 | Scheme 3. Formation of the dioxovanadium (V) complexes with PLSC, PLITSC and PLTS ligands |
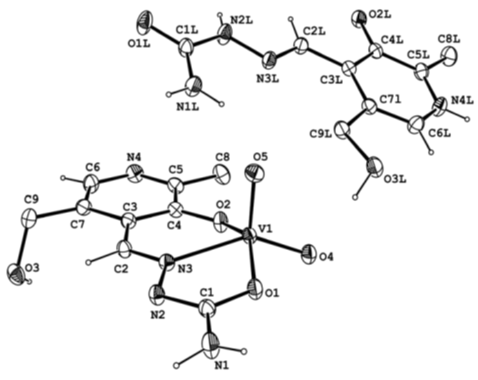 | Figure 2. A view of the anionic complex a NH4 [VO2 (PLSC–2H)] PLSC 2H2O |
 | Figure 3. MERCURY view of [VO2(PLITSC–H)]·2H2O shown with 50 % probability level of the thermal ellipsoids |
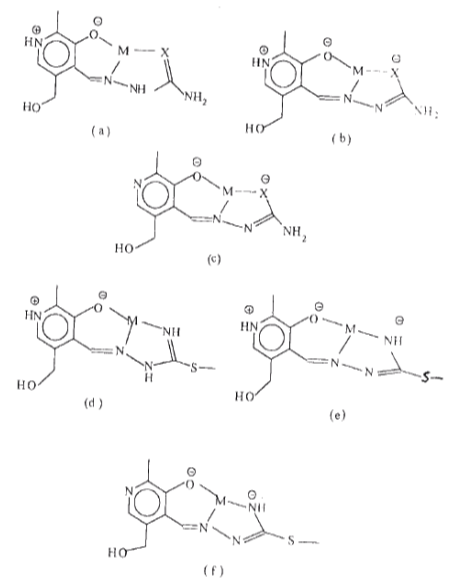 | Scheme 4. The mode of coordination and ligands form for PLSC, PLTSC (X= O or S) (a,b,c) and PLITSC (d,e,f) |
4. Conclusions
- The structure of the title compound C9H12V1N4O5S1, is an interesting metal complex with a Schiff base ligand derived from thiosemicarbazide and pyridoxal (pyridoxal is a 3-hydroxy-5hydroxymethyl-2-methylpyridine-4-carboxaldehyde). Ligand pyridal-thiosemicarbazone (PLTSC; H2L) is a tridentate ONS ligand. The V (V) environment is best described as a square pyramid. The equatorial plane is formed by the tridentate ligand and a two molecule of oxigen. This compound crystallizes in monoclinic symmetry, in space group P 21/c, with lattice constants: a=6.3789(2)Ǻ, b=8.8414(2)Ǻ, c=23.3578(7)Ǻ, α=90°, β=91.3963(11)°, γ=90°, V=1316.95 Å3.
ACKNOWLEDGEMENTS
- The authors acknowledge the Oxford Chemical Crystallography Service for the use of the instrumentation and many thanks to D.J. Watkin research group for help. The use of Nizwa University chemistry labs is highly acknowledge.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML