-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(1): 1-7
doi:10.5923/j.chemistry.20160601.01

Synthesis, Characterization, Thermal Study and Biological Evaluation of Transition Metal Complexes Supported by ONNNO – Pentadentate Schiff Base Ligand
Khalil Abid , Sinann Al – Bayati , Anaam Rasheed
Chemistry Department, College of Science, University of Al – Mustanseriyah, Baghdad, Iraq
Correspondence to: Khalil Abid , Chemistry Department, College of Science, University of Al – Mustanseriyah, Baghdad, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

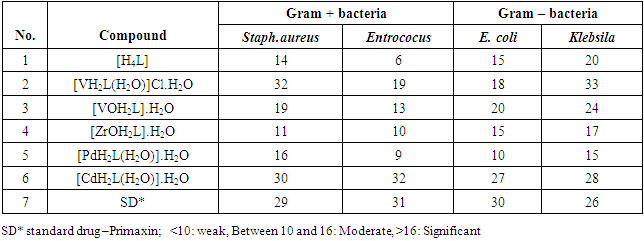
Schiff base ligand H4L [N1,N3-bis(2,4 – dihydroxydeneimino)diethylenetriamine], was synthesised by the condensation reaction of 2,4 – dihydroxybenzaldehyde and diethylenetriamine in 2:1 molar ratio. The ligand which exhibits N3O4 donor set and transitiom metal complexes of VIII, VOII, ZrOII, PdII, RhIII and CdII ions were synthesized and characterized by 1HNMR, fourier transform infrared (FT-IR), UV – vis, mass spectroscopy, elemental analysis (CHNO), thermo gravimetric analysis (TGA), magnetic susceptibility and molar conductivity measurements. Antibacterial activity of the ligand and metal complexes against four kinds of bacteria (two gram + and two gram - ) were investigated.
Keywords: Schiff base, Transition metals, 2,4 – dihydroxybenzaldehyde, N3O4 ligand
Cite this paper: Khalil Abid , Sinann Al – Bayati , Anaam Rasheed , Synthesis, Characterization, Thermal Study and Biological Evaluation of Transition Metal Complexes Supported by ONNNO – Pentadentate Schiff Base Ligand, American Journal of Chemistry, Vol. 6 No. 1, 2016, pp. 1-7. doi: 10.5923/j.chemistry.20160601.01.
Article Outline
1. Introduction
- Schiff base and their 3d – 4f metal complexes containing nitrogen and oxygen donor atoms play an important role in biological and inorganic research and have been studied extensively due to their unique coordination and biological properties. They readily yielding stable and intensely colored metal complexes, some of which have been shown to exhibit interesting physical and chemical properties and potentially useful biological activities [1]. Many Schiff bases have a second functional group, generally an OH, near the imine function. This proximity of the functional groups permits the formation of five or six member chelate rings when coordinated with metal ions. Schiff bases have a diversified structure with nitrogen and oxygen donor systems. Due to the various synthetic procedures, numerous Schiff bases of various structural types have been synthesized [2].Probably the best known Schiff base is N,N′-ethylenebis (salicylideneiminato) or salen, which is a bifunctional and tetradentate (ONNO) ligand. The more general term salen-type is used in the literature to describe the class of [O, N, N, O] bis-Schiff base ligands. The properties of salen-type ligands can be altered by fine-tuning the electronic and steric effects of substituent groups [3, 4]. The doubly deprotonated ligand contains strong donors, namely phenolato oxygen atoms as well as imine nitrogen atoms bearing excellent coordination ability with transition/intertransition metal ions through its N3O2 donor set.Schiff bases are widely used as pigments and dyes, catalysts, intermediates in organic synthesis, and as polymer stabilizers [5]. Schiff bases have also been shown to exhibit a broad range of biological activities, including antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral, and antipyretic properties [6–9].In this paper a new pentadentate dianionic Schiff base ligand, H4L [N1,N3-bis(2,4 – dihydroxy deneimino) diethylenetriamine], that exhibits a N3O4 donor set and some transition metal complexes were synthesized and investigated.
2. Materials and Methods
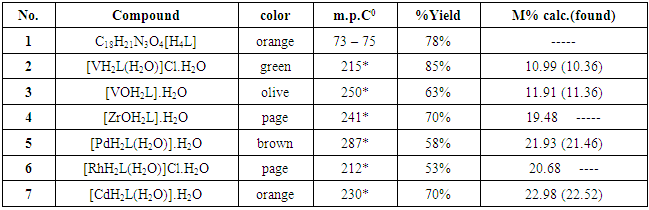
- All chemicals were analytical grade and used without any modification. 1HNMR spectra were obtained using a Bruker 500 MHz and d6 –DMSO as a solvent at University of Al Albait, Amman, Jordan. Fourier transform infrared (FT-IR) spectra were recorded on a Shimadzu spectrophotometer. Elemental analysis for the ligand and metal complexes were carried out using CHNS—elemental analyzer and atomic absorption using Schimadzu model 6809. Mass spectra were recorded by electron impact mass spectrometry (EIMS) using a Direct Injection Probe on a Shimadzu GCMS – QP5050A Spectrometer, Electronic spectra using Varian UV–visible spectrophotometer, Molar conductivity of the complexes were measured in DMSO as a solvent in 0.001M solutions using a CON 510 bench conductivity meter with 2-ring stainless steel conductivity electrode (cell constant, K = 1.0) were carried out at the Chemistry Department, College of Science, Mustanseriyah University, Baghdad, Iraq. Magnetic susceptibility measurements were carried out using the Faraday method with balance magnetic susceptibility model MSB-MKI in the Chemistry Department, College of Science, Al-Nahrain University, Baghdad, Iraq. Thermal stability (weight changes) of the samples was recorded by Mettler Toledo TGA851 and STA 6000 in the temperature up to 1000°C at Ibn-Sina Company, Baghdad, Iraq. Bacterial strains and Mueller Hinton agar were supplied from Department of Biology, College of Science, Mustanseriyah University.Syntheses of H4L [N1, N3-bis(2,4 – dihydroxy deneimino) diethylenetriamine] 2,4 – dihydroxybenzaldehyde (1.12 g, 10 mmol) dissolved in ethanol (20ml) was added to diethylene triamine (0.50 g, 5 mmol) in ethanol (20ml). Few drops of glacial acetic acid was added as catalyst, and then refluxed for 4hrs. The Schiff base ligand was isolated by column chromatography over silica gel (E. Merck) 60–120 mesh size, using a mixture of light petroleum and ethyl acetate (v/v, 1:1). The elute yielded pure was evaporated under reduced pressure to yield a gummy mass, which was dried and stored in vacuum over silica gel for subsequent use (Scheme 1). Yield: 68%, m.p. 73 – 75°C. Elemental analysis for C18H21N3O4, M.wt. 343; calculated (%) C 62.97, H 6.12, N 12.24, O 18.65; found (%) C 63.34, H 6.32, N 12.45, O 18.97.Synthesis of complexes: The appropriate quantity of solid Schiff base ligand H4L (0.686 g, 2 mmol) was dissolved in ethanol (20ml). This ligand solution was added dropwise with stirring to 2mmol of an ethanolic solution of; VCl3, VOSO4.H2O, ZrOCl2, PdCl2, RhCl3 and CdCl2. Adjust the pH to 7 and reflux for 2hr to complete the crystal precipitation process, cooled and filtered. Recrystallization took place from hot ethanol to afford the appropriate precipitate with 53–85% yield, see table 1.
|
3. Results and Discussion
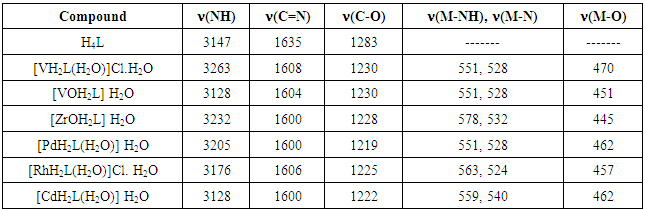
- FT – IR spectra: The (FT-IR) spectra were recorded in the region 4000–400 cm−1 using KBr disc.The ligand (H4L) shows two absorption band at 3570 and 3357cm−1 attributed to υ (OH1) and (OH2) groups respectively. The low frequency for (OH2) is probably due to the considerable amount of H-bonding to the adjacent (>C=N) group [10, 11]. Strong band located at 1639 cm-1 corresponding to imine (C=N) stretching frequency, υ (Ar–O) at 1283 cm−1. Three Bands appeared at 2922 and 1459 cm−1 for aromatic υ (C–H, C=C), and at 2853 cm−1 for aliphatic υ (C–H) [12].The FT-IR spectra of all complexes contain strong peaks characteristic of C=N bands, confirming the complexation of the metal ions. Indeed, upon coordination to the metal center, the imine C=N stretching frequency in the IR spectra dropped from 1639 cm-1 in the free ligand to 1600 – 1608 cm-1 in the complexes. This is further supported by the appearance of new bands at 520 – 530 cm-1 due to υ(M-N) bond. [13]. The presence of coordinated water molecule along with OH1 group was indicated by the appearance of a broad band at 3255 – 3570 cm-1 and two weak bands (for water molecule) in the region 755-787 cm-1 and 709-718 cm-1 due to (-OH) rocking and wagging mode of vibrations, respectively. Ligand coordination to the metal centre is substantiated by another two bands at approximately 550 – 570 and 440 - 455 cm-1 corresponding to υ (M–NH) and υ (M–O) respectively. For VOII complex the infra red spectrum showed an absorption band at 968cm-1 attributed to stretching frequency of V=O [14], while the The zirconyl complexes exhibit one strong band in the region 849 cm-1 which can be attributed to the ν(Zr=O) [15]. Comparison of the infrared spectra of the prepared complexes can lead us to the conclusion that the Schiff base ligand was coordinated in the same manner for all complexes (Table 2).
|
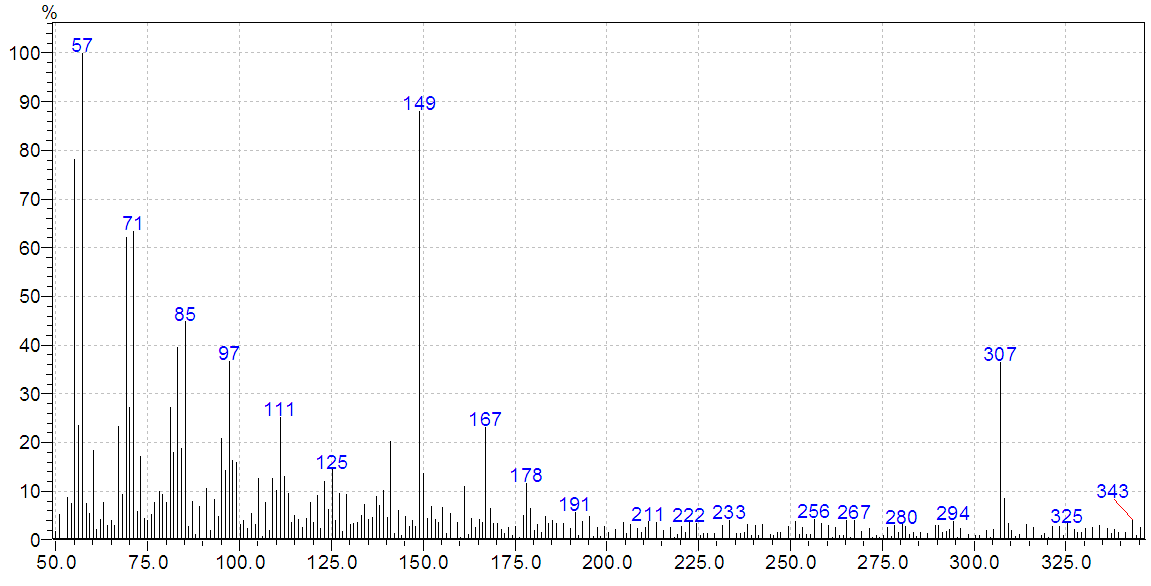 | Figure 1. Mass spectrum of the ligand H4L |
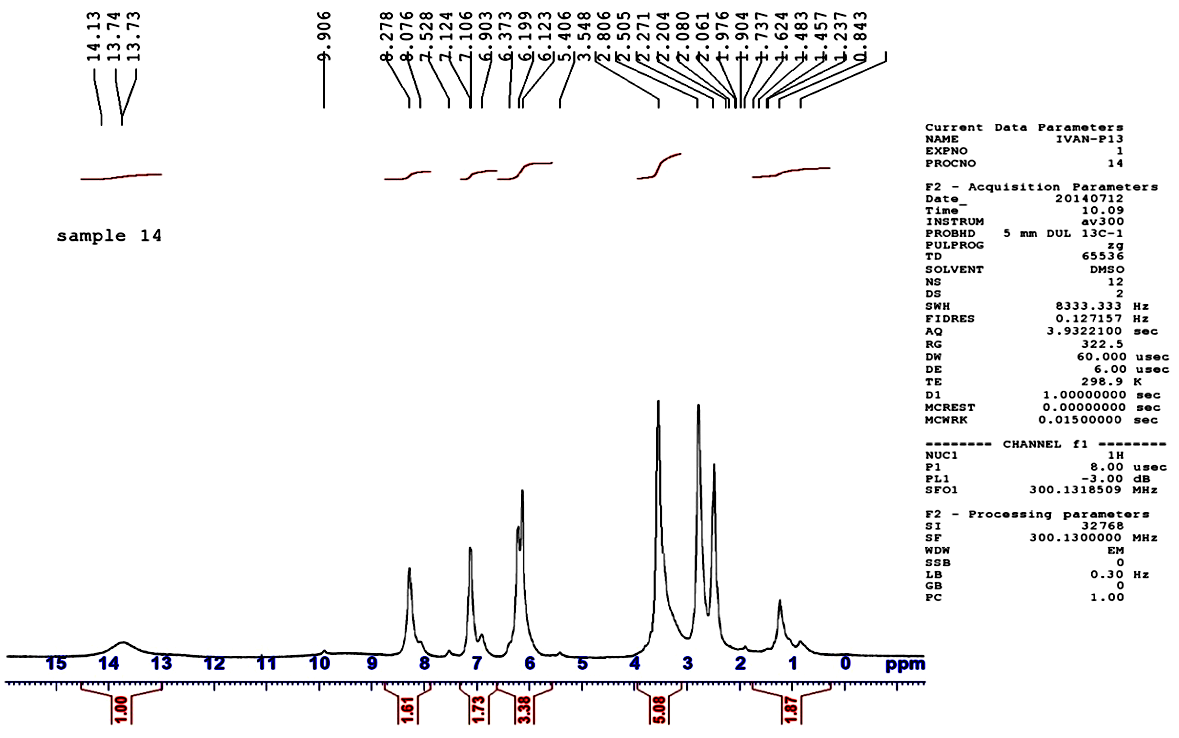 | Figure 2. 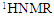 spectrum of H4L spectrum of H4L |
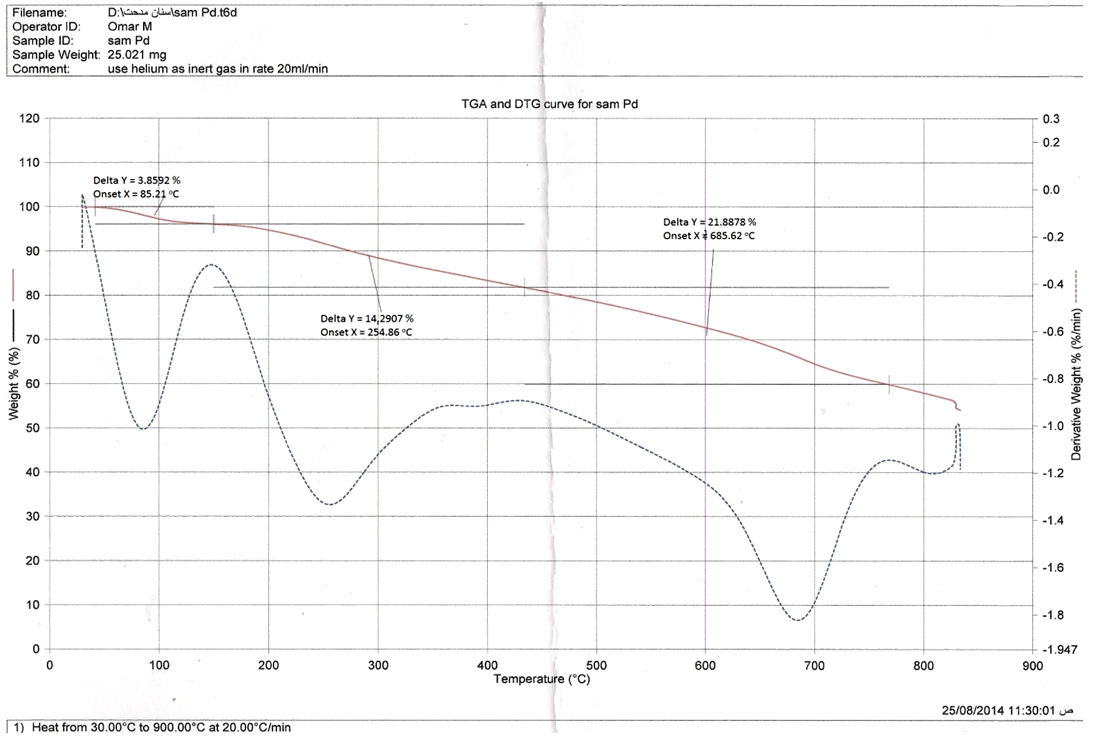 | Figure 3. TGA of 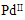 complex complex |
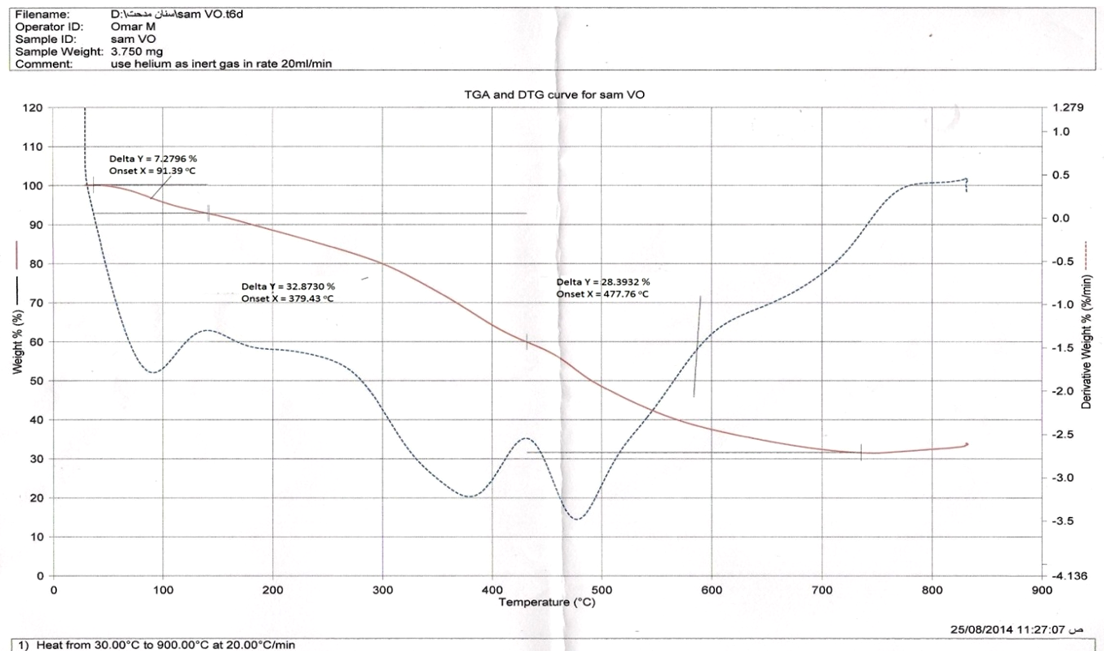 | Figure 4. TGA of  complex complex |
|
4. Conclusions
- An acyclic Schiff base ligand containing N3O4 donor atoms and its mono nuclear transition metal complexes were prepared. The investigation of the analytical data observed suggested the complexes formed with six coordination number as the ligand behaves as pentadentate donor atoms with an additional oxygen atom for VOII, ZrOII and one coordinated water molecule for the others.
ACKNOWLEDGMENTS
- The author would like to thank the University of Al Albait, Amman, Jordan and Ibn-Sina Company, Baghdad, Iraq for spectral data and Al-Mustanseriyah University for providing the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML