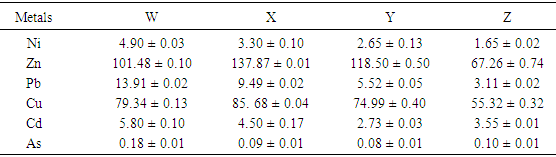-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(5): 125-131
doi:10.5923/j.chemistry.20150505.01

Accumulation of Heavy Metal in Soil and Their Transfer to Leafy Vegetables with Phytoremediation Potential
E. C. Ogoko
Department of Chemistry, National Open University of Nigeria, Lagos, Nigeria
Correspondence to: E. C. Ogoko, Department of Chemistry, National Open University of Nigeria, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

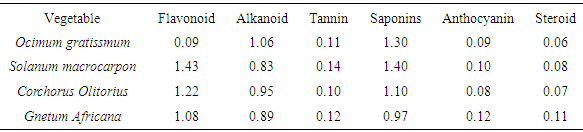
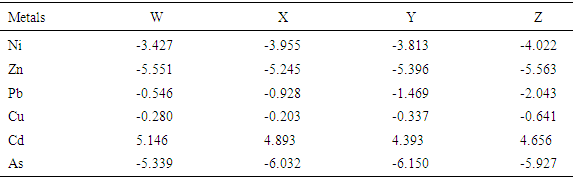
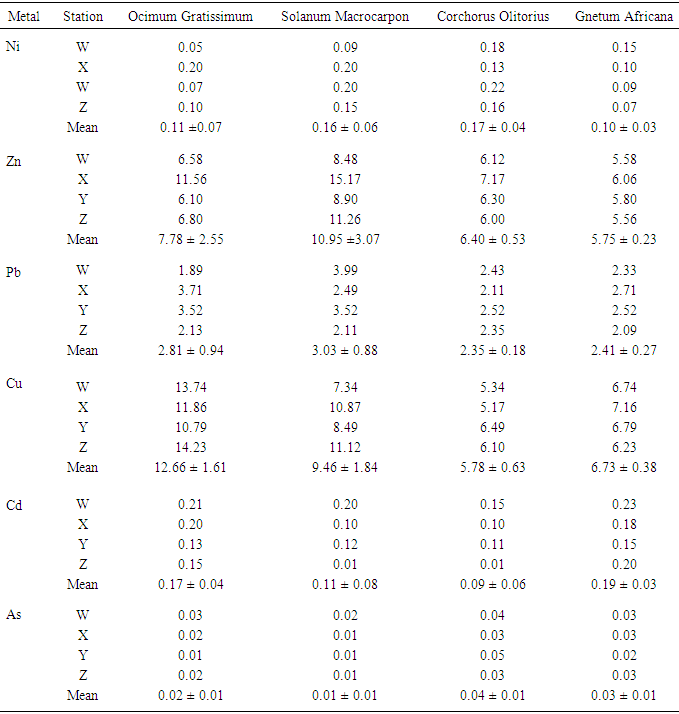
This study attempts to investigate the phytochemical composition, bioconcentration and transfer factors of some selected edible vegetable consumed in Owerri metropolis (Imo State) Nigeria. The concentration of metals in different soils analyzed ranged from 1.65 ± 0.02 to 4.90 ± 0.03 mg/Kg Ni; 67.26 ± 0.74 to 137.87 ± 0.10 mg/Kg Zn; 3.11 ± 0.02 to 13.91 ± 0.02 mg/Kg Pb; 55.32 ± 0.32 to 85.68 ± 0.04 mg/Kg Cu; 2.73 ± 0.03 to 5.80 ± 0.10 mg/Kg Cd and 0.08 ± 0.01 to 0.18 ± 0.01 mg/Kg As. Geo-accumulation index used to established the extent of metal accumulation of the sample stations indicates contamination by cadmium. The levels of metals (Ni, Zn, Pb, Cu, Cd, As) in the various leafy vegetables were within the recommended limits except for lead which was higher than recommended limits, with minimum and maximum values of 2.35 ± 0.18 mg/Kg and 3.03 ± 0.88 mg/Kg respectively. Results revealed that the vegetables investigated were good accumulator of lead, copper and arsenic with significantly high transfer factors which ranged from 0.136 (Ocimum gratissmum) to 0.679 (Solanum macrocarpon), 0.06 (Corchorus Olitorius) to 0.257 (Ocimum gratissmum) and 0.056 (Solanum macrocarpon) to 0.625 (Corchorus Olitorius) respectively. Flavonoid, alkanoid, tannin, saponin and steroid were the phytochemicals detected in the various leafy vegetables investigated, and their concentration ranges from 0.09 – 1.43 g/100g, 0.83 – 1.06 g/100g, 0.11 – 0.14 g/100g, 0.97 – 1.40 g/100g and 0.06 – 0.11 g/100g respectively.
Keywords: Bioaccumulation,Heavy metals, Concentration, Transfer factor, Phytochemical composition
Cite this paper: E. C. Ogoko, Accumulation of Heavy Metal in Soil and Their Transfer to Leafy Vegetables with Phytoremediation Potential, American Journal of Chemistry, Vol. 5 No. 5, 2015, pp. 125-131. doi: 10.5923/j.chemistry.20150505.01.
Article Outline
1. Introduction
- Land is vast and valuable natural resources which sustained the agricultural activities and civilization of mankind. The land has gradually been degraded in an attempt by man to explore and exploit the natural resources endowed in it. The soils are the major reservoir for heavy metal released as a result of vehicular exhaust emission, solid discharge and industrial effluents, gas flaring, insecticides and pesticides, municipal wastes and practices of fertilizer application, spillage of petrochemical and combustion of coal [1]. Chemical elements with specific gravity of at least five times that of water at 39oF are considered to be heavy metals. Mercury, nickel, zinc, copper, chromium, lead, iron, cadmium and arsenic exemplify heavy metals [2, 3]. Heavy metals are not biodegradable and their concentrations vary from soil to soil. Accumulation of heavy metals in the soil over a period of time may lead to excessive uptake of these elements by plants. Bioaccumulation of metals in tissues and on leafs of edible vegetable plants posses serious health risks especially now that there has been increased awareness of the nutritive and medicinal values of leafy vegetables [4, 5]. The presence of elevated levels of trace metals in plants tissues has detrimental toxic effect on animals especially on consumption. For instance, zinc and copper are trace metals because they are needed in minute quantities, though are essential for plant growth as well as animal and human nutrition but at elevated concentrations can lead to phytotoxicity and zootoxicity. [6]. Elevated doses of copper are linked to liver and kidney damage, anaemia as well as irritation of intestine and stomach whereas high doses of zinc in the soil impede the breakdown of organic matter by influencing the activities of microorganism and earthworm on the soil which in turn could result to feeble plant growth and yield depression [7]. Toxic metals such as mercury, cadmium, lead, and arsenic are not essential for either plant or human and animal growth and constitutes risk especially when they go into the food chain [8]. The two main routes of toxic heavy metals exposure to human and other animals are inhalation and ingestion of leafy vegetables, fruits and other food items. Elevated and prolonged lead exposure results to renal damage, death, gastrointestinal tract and central nervous system disorder. Arsenic toxicity is linked to skin damage, circulatory system disorder and high risk of cancer. Nevertheless, mercury is associated with brain, lungs and kidney damage whereas bioaccumulation of cadmium in the kidneys causes permanent damage to the kidney [7, 8].Besides vegetables are good sources of fibers, carbohydrates, vitamins, fats, proteins, minerals, vital amino acids, carotene, saponins, ascorbic acid, steroid, riboflavin, folic acids, iron, calcium, and phosphorus [9, 10]. Some African leafy vegetables have medicinal values and are potent enough to cure more than one disease. Their curative values are thought to be correlated to their phytochemical and other chemical constituents [11]. Studies has shown that high intake of vegetables reduces the risk of diseases of aging due to the presence of sufficient levels of antioxidants as well as phytochemicals in vegetables [12].Metals have excellent ability to be absorbed and assimilated by plants through root and foliar systems. Roots of plants remove metal from soil through filtration, adsorption and cation exchange or stabilization [13, 14]. Different plants species have differing abilities to take in and transport metals at different environmental and soil conditions, and this characteristic is a veritable tool in phytoremediation of soil [15, 16]. This practice involves the use plants to remove and detoxify contaminant in the soil and surface. The process by which certain plants otherwise known as hyperaccumulators, have innate ability to biologically accumulate, degrade and detoxify contaminants in the soil, air and water, is known as Phytoremediation. Interestingly this is prospectively the most cost effective and least detrimental method of getting rid of toxic elements from the environment, though it takes time. Besides it involves the use of naturally occurring organisms thereby mitigating pollutant concentrations and ultimately preserves the environment.The solubility and the kinetics of simple ions adjacent to roots determine greatly the availability of heavy metals to plants in the soil. In this study, the activities of metals (Pb, Cd, Ni, Zn, Cu, As) in both soil and vegetable in Owerri metropolis were investigated.
2. Materials and Methods
2.1. Sample Collection and Preparation
- Owerri is the capital city of Imo State located in the South Eastern Nigeria at Latitude and Longitude of 5° 28’ 59’’N and 7° 01’49’’E respectively (fig 1). Soil samples at depth level (0-30cm) were collected with the aid of soil augar from vegetable farms within the vicinity of Egbu, Amakohia, Orji and Obinze in Owerri Imo State. Plant samples of ocimum gratissimum, solanum macrocarpon, Corchorus Olitorius and Gnetum Africana were also collected from the same farms. The soil samples were labeled W, X, Y and Z. Where W, X, Y, Z represent sampling locations; Egbu, Amakohia, Orji and Obinze (Control) respectively. The soil samples were air dried ground to powder using pestle, mortar and 2 mm mesh sized sieved. The plant samples were thoroughly washed with water and then rinsed with distilled water in order to get rid of contaminant on the surfaces of the leaves and oven dried at 70°C. The dry leaves were weighed and ground into powder for metal concentration analysis.
2.2. Heavy Metal Analysis of the Plant Samples
- The plants samples were digested and analyzed according to the method described by [17, 18]. Previously dry plant samples were extracted by acid digestion using HNO3: H2SO4: HClO4 (10:1:4 v/v). The filtrate was diluted to 50 mL with distilled water followed by heavy metal analysis of the ions of interest using atomic absorption spectrophotometer (FS240) with detection limit of 0.001 ppm. The spectrophotometer was operated under optimal conditions as follows: measurement mode (integrated), Slit with (0.5 nm), gain (79%), lamp current (4.0 mA), flame type (air/acetylene), air flow (13.0 L/min), acetylene flow (2.0 L/min). Certified Reference Material (CTA-OTL-1 Oriental Tobacco Leaves) was used to test the quality of analytical method, using the same quantities of samples. The mean recovery percentages of the reference material were presented in milligrams of metal per kilogram dry weight of sample as followed nickel (103%), zinc (97%), lead (112%), copper (102%), cadmium (117%) and arsenic (99%).
2.3. Heavy Metal Analysis of the Soil Samples
- Total concentration of some heavy metals of interest was determined according to the method of Lokeshwari and Chandrappa [19]. Weigh accurately 2g each of soil samples in a 250 ml glass beaker followed by digestion with 8 mL of aqua regia on a sand bath for 120 minutes. The sample was evaporated to dryness and then dissolved with 10 mL of 2% nitric acid, filtered and diluted to 50 ml with deionized water. The filtrate was measured of total concentrations of the heavy metals of interest using atomic absorption spectrophotometer (FS240) and conditions as described above.
2.4. Phytochemical Composition of Plant Samples
2.4.1. Determination of Flavonoid Content
- Flavonoid in plant samples was determined according to the method described by Harborne [20]. Weigh accurately 5 g of plant sample in a round bottom flask and then boiled and refluxed in 50 mL of 2 M HCl solution for 35 minutes. The mixture was allowed to cool followed by filtration using whatman filter paper. The filtrate was treated with equal volume of ethyl acetate drop wise until precipitation was completed. The precipitate was recovered by filtration using a previously weighed filter paper. The weight of the precipitate was measured and recorded.
2.4.2. Determination of Alkaloids
- This was performed by gravimetric method described by Harborne [20]. 5g of plant sample was dispersed in 10% acetic acid solution in ethanol (1:10). The mixture was allowed to stand for 4 hours at 28°C, filtered, washed with 1% ammonia solution and dried in an oven at 80°C. Alkaloid content was then calculated and expressed as a percentage of the weight of plant sample analyzed.
2.4.3. Determination of Tannin
- 500 mg of the plant sample was weighed into 100 mL plastic bottle followed by addition of 50 ml of distilled and then shaken for 1 h using a mechanical shaker. The mixture was filtered into a 50 ml volumetric flask and diluted up to the mark. 5 ml of the filtrate was then pipette into a cuvette followed by addition of 3 ml of 0.1 M FeCl3 in 0.1 N HCl and 0.008 M potassium ferrocyanide. The content of the cuvette was thoroughly mixed and the absorbance was taken at 120 nm wavelength using a spectrophotometer. The blank sample was prepared and measurement made at the same wavelength. Tannin acid was used in the preparation of 100 ppm standard solution and absorbance measured [21].
2.4.4. Determination of Saponins
- 20 g of ground plant sample was dispersed in 200 ml of 20% ethanol. The mixture was heated in a hot water bath for 4 h with vigorous stirring at 55°C followed by filtration. The residue was re-extracted with another 200 ml of 20% ethanol. The combined extracts were concentrated to 40 ml by evaporation in a water bath at 90°C. 20 ml of diethyl ether was added to the concentrate in a 250 ml separating funnel with vigorous shaking. The ether layer was discarded while the aqueous layer was recovered followed repetition of the purification process with 60 ml of n-butanol. The combined n-butanol extracts were washed with 10 ml of 5% aqueous NaCl then evaporated in water bath, dried in an oven to a constant weight. The % saponin content was then calculated [22].
2.4.5. Determination of Anthocyanin
- 5 g of each plant sample was boiled in 100ml of 2M HCl solution for 35 minutes followed by filtration of the hydrolysate using whatman No 42 filter paper. Ethyl acetate was poured into a previously washed separation funnel which contains the filtrate. The content of the separating funnel was thoroughly mixed and then allowed to separate into two distinct layers. The extract (ethyl acetate layer) noted and evaporated to dryness in a crucible over a steam bath. Concentrated amyl alcohol was added to the dried extract in other to release the anthocyanins followed by filtration. The combined filtrate and alcohol extract were transferred to an evaporating dish and evaporated to dryness, dried in the oven at about 30°C for 30 min and cooled. Then the % anthocyanin content was calculated and adequately recorded [20, 23].
2.4.6. Determination of Steroid Content
- 10 g of each sample was dissolve in 100 ml distilled water with vigorous stirring and blended by the aid of a laboratory blender. The supernatant solution was filtered using whatman No 42 filter paper and then ammonium hydroxide solution was used to elute the filtrate at pH 9.3ml of chloroform was mixed with 3 ml of the eluate in a previously washed test tube. 5ml of acetic anhydride (ice-cold) were poured into the mixture of chloroform and eluate in a clean round bottom flask and 3 drops of concentrated tetraoxosulphate (vi) acid were as well added. Similarly a standard solution of sterol was prepared and treated as described previously. The absorbance of both prepared sample and the standard solution was measured at 420 nm using a spectrophotometer [24].
3. Results and Discussion
- Table 1 represents the phytochemical composition of some leafy vegetables cultivated in South-eastern Nigeria.
|
|
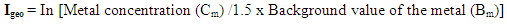 | (1) |
|
|
|
4. Conclusions
- The result of this study revealed the presence of phytochemicals (Flavonoid, Alkanoid, Tannin, Saponins, Anthocyanin and Steroid). Various heavy metals (Cu, As, Ni, Pb, Zn and Cu) were detected in the various plants at levels which do not pose serious health risks to the unsuspecting consumers except for lead whose concentrations were above recommended values (2 mg/Kg) by WHO/FAO as well as NAFDAC. Metals with very high concentrations tend to have very low TF values indicating greater retention of metals in the soil. This phenomena observation indicated also greater efficiency of vegetables to absorb metals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML