-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(4): 115-123
doi:10.5923/j.chemistry.20150504.03

Exfoliated Zirconium Phosphate-Fibrous Cerium Phosphate Nanocomposite Membrane Self Supported Indole, Its Co-Aniline and Co-Pyrrole Polymerization Agent
S. K. Shakshooki, F. A. Elakari, Aisha M. Shaabani
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: S. K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Nanosized zirconium phosphate and nano fibrous cerium phosphate, Zr(HPO4)2.H2O(nZrP), Ce(HPO4)2.2.9H2O (nCePf), respectively, were prepared and characterized. Mixing slurry aqueous solution of (nZrP and nCePf) in 25:75 wt/wt% mixing ratios), respectively, lead to formation of novel zirconium phosphate- fibrous cerium phosphate nanocomposite membrane, [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75.3.87H2O(nZrP-nCePf), and was characterized. Zirconium phosphate-fibrous cerium phosphate / polyindole, - / polyindole-co-polyaniline and -/polyindole-co-polypyrrole nanocompsite membranes were prepared via in-situ chemical oxidation of the indole, and its co-monomers, that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix of (nZrP-nCePf ) nanocomposite membrane. A possible explanation is nCePf present on the surface of the fiber is attacked by indole and its co-monomers, converted to cerium (III) orthophosphate(CePO4). The resultant materials were characterized by elemental (C,H,N) analysis, FT-IR, and scanning electron microscopy(SEM). The rest nanocomposite membranes morphology was preserved. SEM images of the resulting nanocomposites reveal a uniform distribution of the polymer and co-polymers on the inorganic matrix. From elemental (C,H,N) analysis the amount of organic material present in (nZrP-nCePf)/PIn composite found to be (8.83% in wt.). Where the amount of organic material present in (nZrP-nCePf)/PIn-co-PANI found to be PIn =17.51, PANI = 26.26% in wt., and for (nZrP-CePf) / PIn-co-PPy found to be PIn = 21.84, PPy = 29.13 % in wt..
Keywords: Exfoliated zirconium phosphate-cerium phosphate nanocomposite membrane, Self support polymerization, indole, Indole-Co-Aniline and indole-co-pyrrole
Cite this paper: S. K. Shakshooki, F. A. Elakari, Aisha M. Shaabani, Exfoliated Zirconium Phosphate-Fibrous Cerium Phosphate Nanocomposite Membrane Self Supported Indole, Its Co-Aniline and Co-Pyrrole Polymerization Agent, American Journal of Chemistry, Vol. 5 No. 4, 2015, pp. 115-123. doi: 10.5923/j.chemistry.20150504.03.
Article Outline
1. Introduction
- Electronically conducting polymers exhibit wide range of electrical conductivity from semiconductors to metallic region by way of doping [1, 2], called as synthetic metals, have been the subject of great interest in recent years because of their enormous interesting properties like high electrical conductivity, environmental and chemical stability, low cost, easy prepared by chemical oxidative polymerization and electrochemical methods and fast reversible doping and dedoping [1-4]. Conducting polymers such as polyaniline (PANI), and polypyrrole (PPy) have received increasing attention in various fields. Due to their excellent properties they can be used in various applications like solar cells, sensors, batteries, and super capacitors [1-8] in electronics, industry and others [1, 5, 6]. Their electrical and electrochemical properties show great promise for commercial applications. However; among various aromatic compounds-based conducting polymers polyindole(PIn) and its derivatives has been less investigated although there exists close structural similarities with the polymers mentioned above [4]. Polyindole can be obtained from chemical, electrochemical and interfacial polymerization of indole [2, 3, 5, 6]. Its electrical and electrochemical properties show great promise for commercial applications [5-10]. Polyindole is an electroactive polymer, owns advantages especially fairly good thermal stability [5, 6]. Some studies shows polyindole has similar properties like polyaniline, based on their high conductance and good environmental stability [2, 4, 11-13]. polyaniline (PANI) and polypyrrole (PPy) show ease of synthesis by chemical and electrochemical method of polymerization. Out of these two methods, chemical polymerization is preferred because of its low cost, efficiency and mass production over electrochemical method [14]. Polyanilinne and heterocyclic Conducting polymers are an air-stable organic materials, which are relatively easy to polymerize and to control its electrical conductivity.Inorganic layered tetravalent metal phosphates nanomaterials are receiving great attention because of their size, structure, and possible biochemical applications [14, 15], that have been proven to be good carriers for organic polar molecules. These materials are good thermal stability and des not change on aging. Examples of these are zirconium phosphates. Taking advantage of the expandable of their layered. Cerium phosphates have been studied for a long time as ion exchangers, their structures remains unknown until recently [16, 17, 18]. The reason is that, the composition, the structure and the degree of crystallinity of their precipitates results from reaction of solutions containing a CeIV salt is mixed with a solution of phosphoric acid of [(PO4)/CeIV ratio], strongly dependent on the experimental conditions such as rate and order of mixing of the solutions, stirring, temperature and digestion time, this also implemented on fibrous cerium phosphate [19]. To date most of the work on fibrous cerium phosphate was carried out on its ion exchange [20], intercalation [21] and electrical conductance properties [22]. Studies on its polystyrene, polyacrylamide [23] and (polyvinyl chloride-based polyvinylalcohol) [24] composites have been reported. Nanoscaled tetravalent metal phosphates and their organic polymer composites comprise an important class of synthetic engineering. However; research in such area is still Terra incognita [25-28]. Nanotechnologies are at the center of numerous investigations and huge investments. Chemistry has anticipated for long the importance decreasing the size in the search of new properties of materials, and of materials structured at the nanosize in a number of applications relate to daily life. Organic-inorganic nanocomposite membranes have gained great attention recently [28, 29]. The composite material may combine the advantage of each material, for instance, flexibility, processability of polymers and the selectivity and thermal stability of the inorganic filler [26-30]. Conducting polymers are interesting materials owing to their electrical properties [25, 27]. Belonging to this class are polyaniline, polypyrrole, polyindole, polybenzimidazole and others. Recently a great deal of attention has been paid towards synthesis of conducuing co-polymers because the co-polymer provides higher properties than the individual conducting polymer. It has been observed experimentally that copolymerization of two different monomers increases the number of conductive polymers which cannot be achieved by using single monomer [31]. The composites of conducting polymers can be synthesized by different methods like in-situ chemical polymerization method, electrochemical polymerization, mechanical mixing of the two substances and microwave assisted synthesis. The synthetic methodologies of the composite greatly affect different properties of the composites [31-33]. In our laboratory we are carrying systematic investigations on novel tetravalent metal phosphates / organic heterocyclic conducting polymers nanocomposite membranes. Recently we have reported [34-36] the preparation and characterization of fibrous cerium phosphate/ polybenzimidazole [34], / polyindole [35], / polyaniline [36] nanocomposite membranes. The present study describes the preparation and characterization of novel zirconium phosphate-fibrous cerium phosphate nanocomposite membrane, [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O, supported Indole-, its co-aniline-, and its co-pyrrole polymerization agent via in-situ chemical oxidation that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix [34-36].
2. Materials and Methods
2.1. Chemicals
- Ce(SO4)2.4H2O, ZrOCl2.8H2O, H3PO4(85 %) of BDH, indole of Reidel de-Haen , aniline (99.5%) of Mindex UK, pyrrole of Aldrich. Other reagents used were of analytical grade.
2.2. Instruments Used for Characterization
- X-Ray powder diffractometer Siemens D-500, using Ni-filtered CuKα (λ=1.54056Å), Thermogram C-MOM-Budapest, TG/DTA SIIExtra6000 TGA Perkin Elmer thermo gravimetric analyzer (TGA7)US, CHN-Elmental analyzer, Vario Elemental-German. Fourier Transform IR spectrometer, model FT/IR-6100, Scanning electron microscopy (SEM) Jeol SMJ Sm 5610 LV. Transmission electron microscopy) (TEM) Zeiss 10CR and pH Meter WGW 52.
2.3. Preparation of Nanofibrous Cerium Phosphate Membrane, Ce(HPO4)2.2.9H2O (nCePf)
- Nanofibrous cerium phosphate membrane was prepared from adding 300 ml of 0.05M CeSO4.4H2O in 0.5 M H2SO4 solution, drop wise, to 300 ml of 6 M H3PO4 at ~ 80C with stirring. After complete addition the resultant material left to digest at that temperature for 4h. To that 3 liter of hot distilled water, (~60C), was added with stirring for 1h. The resultant slurry aqueous solution of nanofibrous cerium phosphate was kept. The sheet form of nanofibrous cerium phosphate membrane can be obtained by filtration of the resultant slurry aqueous solution of nanofibrous cerium phosphate on Buchner funnel.
2.4. Preparation of Nanozirconium Phosphate, Zr(HPO4)2.H2O(nZrP)
- Novel layered nanosized zirconium phosphate was prepared from refluxing 35 grams of its parent wet gel Zr(HPO4)2.nH2O, (where n = ~75% content ) in 145 ml 2M H2PO4 for 50 hours. The resultant product was washed with distilled water up to pH3, filtered on Buchner funnel, then dried in air.
2.5. Preparation of Exfoliated Zirconium Phosphate-Fibrous Cerium Phosphate Nanocomposite Membrane
- Composite membrane [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O(nZrP-nCePf) was prepared by mixing nZrP and nCePf in wt/wt % ratio, 25:75% of nZrP: nCePf (0.15g nZrP0 and 150ml slurry aqueous of nCePf (0.45g). The mixing was carried out at 75°C with stirring for 48h. The resultant product was filtered on filter paper using Buckner funnel, washed with distilled water (50ml) twice and dried in air. The products were homogeneous flexible thin film.
2.6. Preparation of Zirconium Phosphate-Fibrous Cerium Phosphate/Polyindole Nanocomposite Membrane
- zirconium phosphate-fibrous cerium phosphate/ polyindole nanocompsite membrane was prepared by addition of 6 ml 4% indole in ethanol, to 33mg of nZrP dispersed in 36ml of the original slurry aqueous solution of fibrous cerium phosphate (nCePf content = 100mg), at room temperature with stirring for 3h(nCePf , then kept static at room temperature for 48h. It was observed with time the color changes gradually to (pale brown and finally to dark green). The resultant composite was filtered, washed with distilled water and ethanol and left to dry in air
2.7. Preparation of Zirconium Phosphate-Fibrous Cerium Phosphate/Polyindole-Co-Polyaniline and Polyindole-Co-Polypyrrole Nanocomposite
- In similar manner zirconium phosphate-fibrous cerium phosphate/polyindole-co-polyaniline nanocompsite membrane and polyindole-co-polypyrrole nanocomposite membrane were prepared by additionof 4% 7.5ml aniline and 7.5ml indole in ethanol [or 4% 7.5ml indole and 4% 7.5ml pyrrole in ethanol], respectively, to 50mg of nZrP dispersed in 55 ml of the original slurry aqueous solution of fibrous cerium phosphate, (nCePf content = 150mg), at room temperature with stirring for 3h, then kept in fridge for48h. The resultant composites were filtered, washed with distilled water and ethanol and left to dry in air. Their color were green and blackish green, respectively.
2.8. Separation of Polyindole-Co-Polyaniline and Polyindole-Co-Polypyrrole from Their Composites by HF Solution
- To 0.15g of each of zirconium phosphate-fibrous cerium phosphate/polyindole-co-polyaniline (and zirconium phosphate-fibrous cerium phosphate / polyindole-co-polypyrrole) nanocomposite membranes, in plasic container, 15ml of 6M HF solution was added that subjected to stirring for 24h. The remaining material, the copolymers, were collected by filtration, washed with distilled water and ethanol and allowed to dry in air.
2.9. Exchange Capacity
- Exchange capacities of the Nanosized M(IV) phosphates were determined by addition of 25 ml of 0.1M NaCl solution to 100 mg of the material, with stirring for one hour , then titrated with 0.1 M NaOH solution.
3. Results and Discussion
- Nanofibrous cerium phosphate membrane, Ce(HPO4)2. 2.9H2O (nCePf), was prepared, characterized by chemical, XRD, TGA, FT-IR, scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
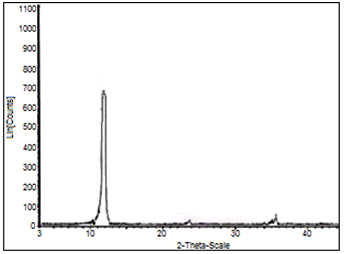
3.1. XRD
- Nanosized fibrous cerium phosphate, Ce(HPO4)2.2.9H2O, was obtained via reflux method . The fibrous nature can be visually recognized. ItsXRD of is shown in Figure 1, with d001 = 10.89Å.
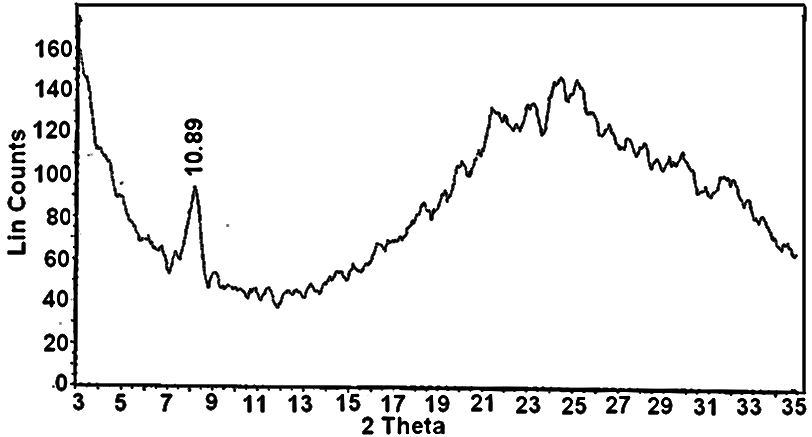 | Figure 1. XRD of nanofibrous cerium phosphate |
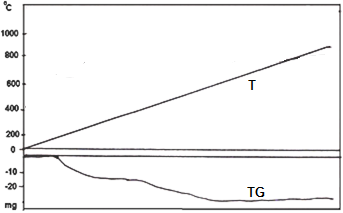
3.2. TGA
- Thermogram of Ce(HPO4)2.2.9H2O is shown in Figure 2. The thermal decomposition occurs in continuous process almost one step. The thermal analysis was carried out at temperatures between 10-775°C, the final product was CeP2O7, results from the loss of water of hydration between 60-200°C, followed by POH groups condensation. The total weight loss found to be equal to 19.09%.
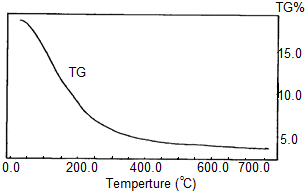 | Figure 2. TGA of nanofibrous cerium phosphate |
3.3. FT-IR
- Figure 3 shows FT-IR spectrum of fibrous Ce(HPO4)2.2.9H2O, with a trend similar to that of M(IV) phosphates. It consists of broad band centered at 3350cm-1 is due to OH groups symmetric stretching of H2O, small sharp band at 1628cm-1 is related to H-O-H bending. Sharp broad band centered at 1045cm-1 is corresponds to phosphate groups vibration The bands at the region 630-450 cm-1 are ascribe the presence of δ(PO4).
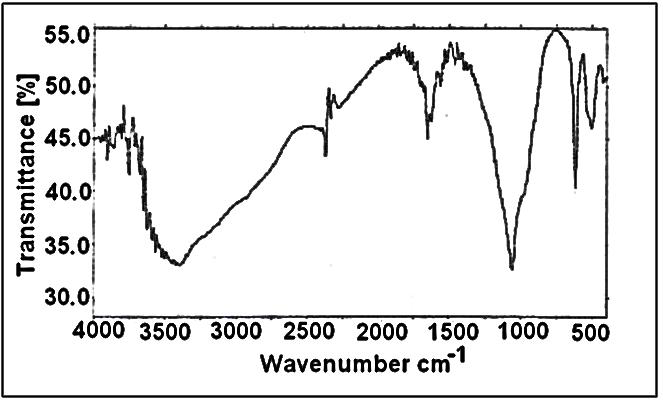 | Figure 3. FT-IR spectra of nanofibrous cerium phosphate |
3.4. SEM
- SEM morphology image of the nanosized fibrous cerium phosphate shown in Figure 4. The photograph shows its average size is ~20.5 nm.
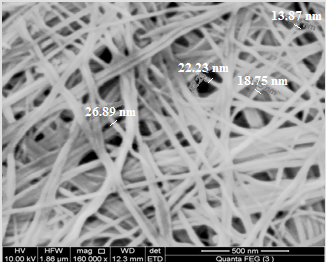 | Figure 4. SEM morphology image of (nCePf) |
3.5. TEM
- Transmission electron microscopy image (TEM) of the nanosized fibrous cerium phosphate , of fibrous visual look, is shown in Figure 5. The photograph shows its average size is ~15nm.
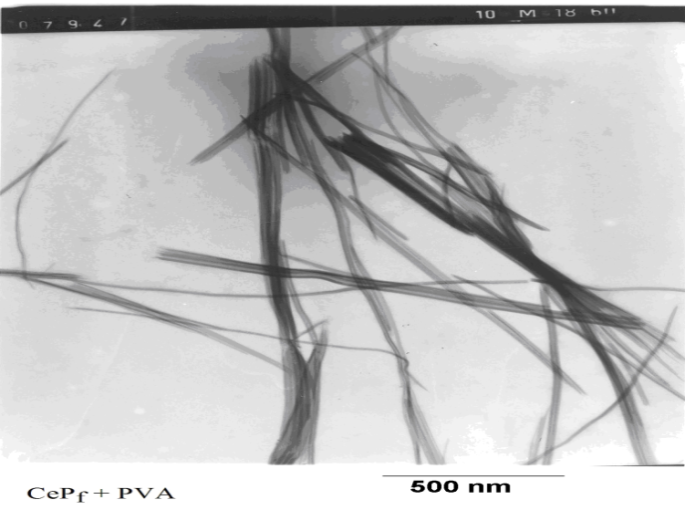 | Figure 5. Miograph image (TEM) of (nCePf) composite thin film in PVA matrix |
3.6. XRD of nZrP
- The exfoliated nZrP formed upon refluxing wet gel of ZrP. This result lead to formation pellicular type membrane. Its XRD is shown in Figure 6. with d001 7.65 Å. XRD shows the material consists mainly of layers oriented parallel to its surface, so its x-ray diffractogram peaks are similar to that of a highly iso-oriented samples of its parent crystalline layered material.
 | Figure 6. XRD of nZrP |
3.7. TGA of nZrP
- Thermogram of exfoliated Zr(HPO4)2 .H2O(nZrP), is shown in Figure 7. The thermal analysis found to occur in two stages. The first stage is related to the loss of water of hydration between 70-200°C, followed by POH groups condensation. The total weight loss found to be equal to 12%. The final product was ZrP2O7.
 | Figure 7. TGA of nZrP |
3.8. FT-IR of nZrP
- Figure 8 shows FT-IR spectrum of Zr(HPO4)2.H2O, with a trend similar to that of M(IV) phosphates. It consists of broad band centered at 3350cm-1 is due to OH groups symmetric stretching of H2O, small sharp band at 1628cm-1 is related to H-O-H bending. Sharp broad band centered at 1045cm-1 is corresponds to phosphate groups vibration The bands at the region 630-450 cm-1 are ascribe the presence of δ(PO4).
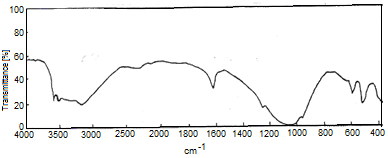 | Figure 8. FT-IR spectrum of nZrP |
3.9. SEM of nZrP
- SEM morphology image of the exfoliated nanosized zirconium phosphate (nZrP) is shown in Figure 9. The photograph shows its average size in the range 48nm.
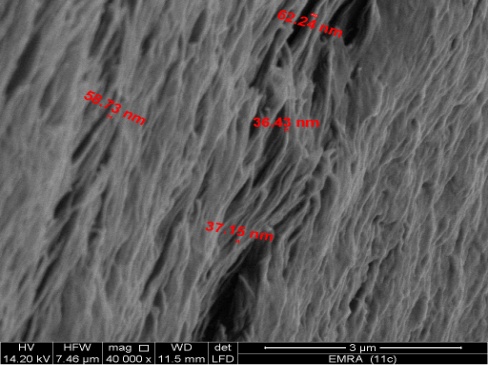 | Figure 9. SEM morphology image of (nZrP) |
3.10. TEM of nZrP
- Transmission electron microscopy image (TEM) of the nanosized zirconium phosphate, is shown in Figure 10. The photograph shows formof multi particle aggregates of average size of the smallest ones ~80nm.
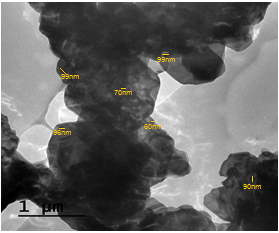 | Figure 10. Miograph image (TEM) of (nZrP) |
3.11. XRD of nZrP-nCePf Nanocomposite Membrane
- X-ray diffraction of nanocomposite membrane, [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75.3.87H2O, is shown in Figure 11, shows two major peaks of 11.31Å and 7.81Å which are related to the interlayer distance of their parent materials which shows the formation of the composite. The first d value concern nCePf, the second one is related to nZrP.
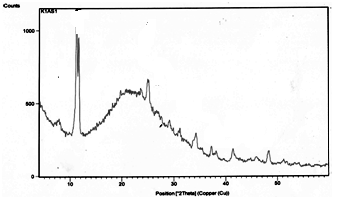 | Figure 11. XRD of [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75.3.87H2O |
3.12. TGA of nZrP-nCePf Nanocomposite Membrane
- Thermogram of [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75.3.87H2O is shown in Figure 12. The thermal analysis found to occur in three stages. The first stage is related to the loss of water of hydration between 70-240°C, followed by POH groups condensation up to 800°C. The total weight loss found to be equal to 22.51%. The final product was [Zr0.25- CeP 0.75]P2O7 The thermogra is accomponed with three endothermic peaks.
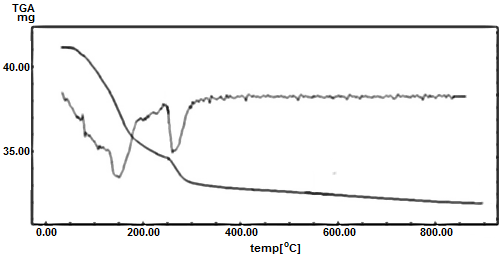 | Figure 12. TGA/DTA of [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O |
3.13. SEM of nZrP-nCePf Composite Membrane
- Figure 13 showed that the surface morphology of nanocomposite membrane [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O is totally different from their individual inorganic components.The morphology image reveal a uniform distribution of nZrP over and between fibrous cerium phosphate matrix.
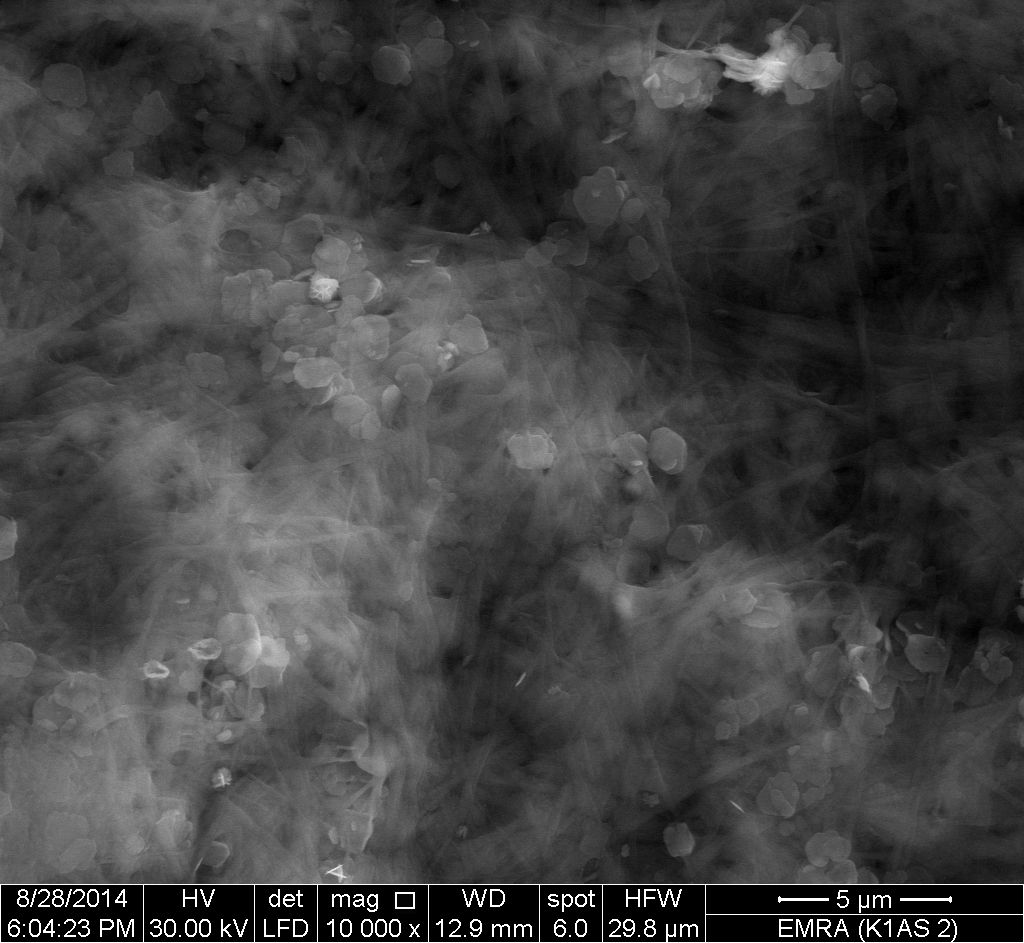 | Figure 13. SEM morphology image of [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O composite |
3.14. (nZrP-nCePf)/Polyindole Nanocomposite Membrane
- It was found that when [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75. 3.87H2O immersed in ethanolic solution of indole the color of self supported sheet gradually changes with time to (pale brown and finally to dark green). The resultant nanocomposite membrane was characterized by elemental (C, H, N) analysis TGA and by FT-IR spectroscopy.From elemental (C, H, N) analysis, the amount of organic material present in the (nZrP-nCePf)/polyindole nanocomposite membranes was found to be 8.83% in weight. The % of elemental (C, H, N) related to the organic polymer found to be C = 6.286%, H = 0.449 %, N = 2.095%.A possible explanation is that polymerization of indole was promoted by the reduction nCePf present on the surface of the fiber is attacked by indole converted to cerium(III) orthophosphate(CePO4).It is important to note that the self-supported sheet integrity is preserved. This result is interesting because the shape integrity makes the building of molded conducting device possible.
3.14.1. SEM of (nZrP-nCePf)/Polyindole Nanocomposite Membrane
- Figure 14 shows SEM image for (nZrP-nCePf)/polyindole nanocomposite membrane, reveal a distribution of the polymer on the inorganic matrix (nZrP-nCePf). The inorganic matrix show the morphology image reveal a uniform distribution of nZrP over and between fibrous cerium phosphate matrix.
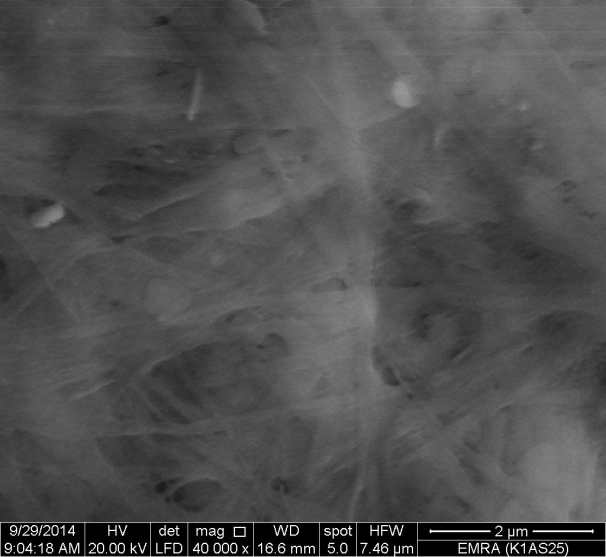 | Figure 14. SEM morphology image of (nZrP-nCePf)/polyindole nanocomposites |
3.14.2. FT-IR of (nZrP-nCePf)/Polyindole Nanocomposite Membrane
- Figure 15. shows typical FT-IR spectrum of (nZrP-nCePf)/polyindole nanocomposite membrane. It consists of broad band centered at 3429 cm-1 is due to OH groups symmetric stretching of H2O, small sharp band at 1625 cm-1 is related to H-O-H bending, and sharp broad band centered at 1045 cm-1 is corresponds to phosphate groups vibration. Small bands at 2925 and 2857cm-1 could be attributed to N-H stretching. Bands in the range 1893-1600cm-1 are related to stretching C-C bonds characteristic of indole unites C-H (aromatic) stretching, C=C stretching. C-N stretching (between two indole units), are in the region 1600-1370 cm-1 [9].
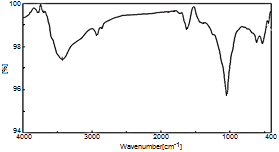 | Figure 15. FT-IR spectrum of (nZrP-nCePf)/polyindole nanocomposite membrane |
3.15. (nZrP-nCePf)/ / Polyindole-Co-Polyaniline and -/Polyindole-Co-Polypyrrole Nanocomposite Membranes
- On immersing of [Zr(HPO4)2]0.25[Ce(HPO4)2]0.75.3.87H2O nanocomposite membrane in ethanolic solution of indole-co-monomers (aniline, pyrrole), the color of self supported sheet gradually changes with time to green and blackish green , respectively. A possible explanation is that polymerization of indole-comonomers were promoted by the reduction nCePf present on the surface of the fiber is attacked by indole-co-monomers, converted to cerium (III) orthophosphate (CePO4). Self-supported sheet integrity was preserved.The resultant materials were characterized by elemental (C,H,N) analysis, FT-IR, and scanning electron microscopy (SEM). From elemental (C,H,N) analysis the amount of organic material present in (nZrP-nCePf)/ PIn-co-Pani found to be PIn = 17.51, PANI = 26.26 % in wt. , [the C, H, N analysis, of the copolymer, found to be C= 36..31, H= 1.696 and N = 5.80% in wt].For (nZrP-CePf) / PIn-co-PPy found to be PIn = 21.84, PPy = 29.13 % in wt.., [the C, H, N analysis, of the copolymer, found to be C= 41.92, H = 2.38 and N = 6.64% in wt]. [S found to be 0.12 and 3.18% in wt, respectively].From the elemental C,H, N analysis, the ratios of each polymer in the co-polymer was calculated.The total % in wt of the co-polymers from CHN analysis found to be in agreement with the contents of copolymers that recovered from HF solution experiment. The inorganic (nZrP-nCePf) readily dissolves in HF solution, the remaining were the copolymers.
3.15.1. SEM of (nZrP-nCePf)/Polyindol-Co-Polyaniline, / Polyindole-Co-Polypyrrole Nanocomposite Membrane
- Figures (16, 17) show SEM images for (nZrP-nCePf) / polyindole-co-polymers naoncomposite membranes, reveal a uniform distribution of the co-polymers on the inorganic matrix (nZrP-nCePf). As can be seen the size and the shape of the copolymers are depend on the organic monomers used. Spherical particles were obtained with monomer pyrrole [35], as shown in Figure 17.
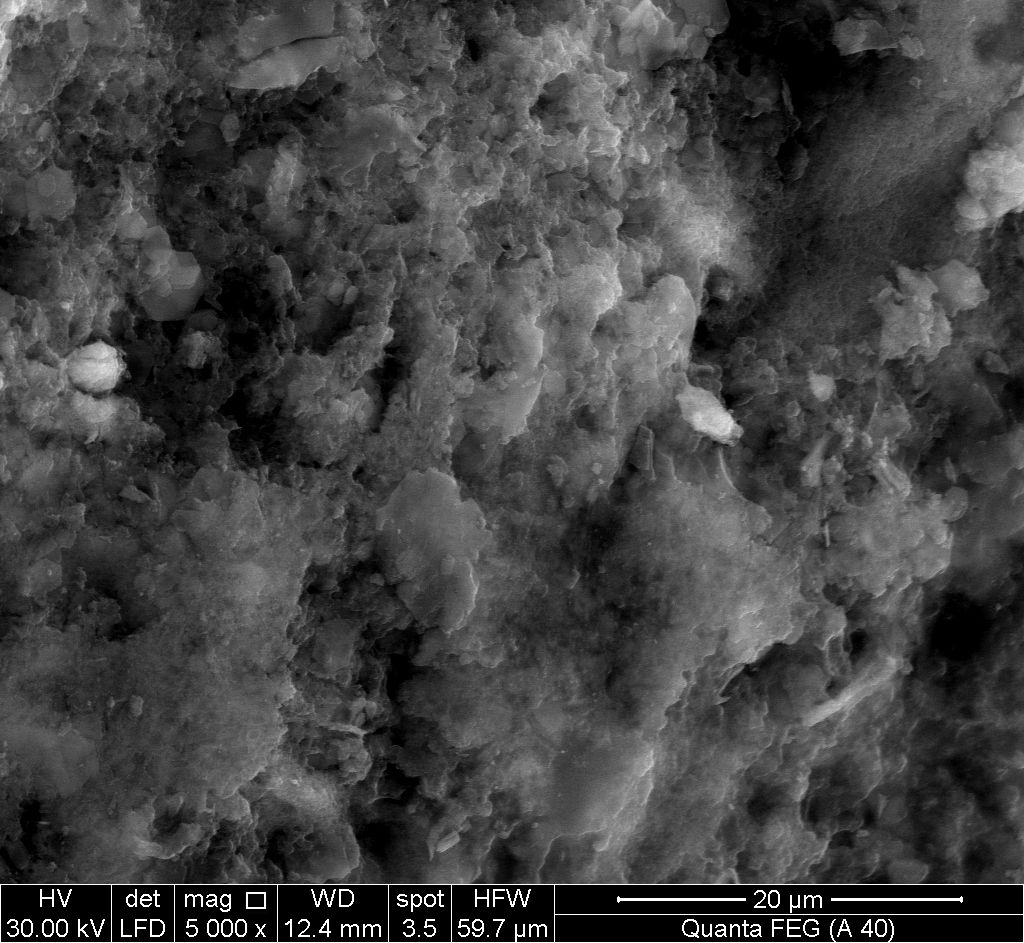 | Figure 16. SEM morphology image of (nZrP-nCePf)/ polyindole-co-polyaniline nanocomposite |
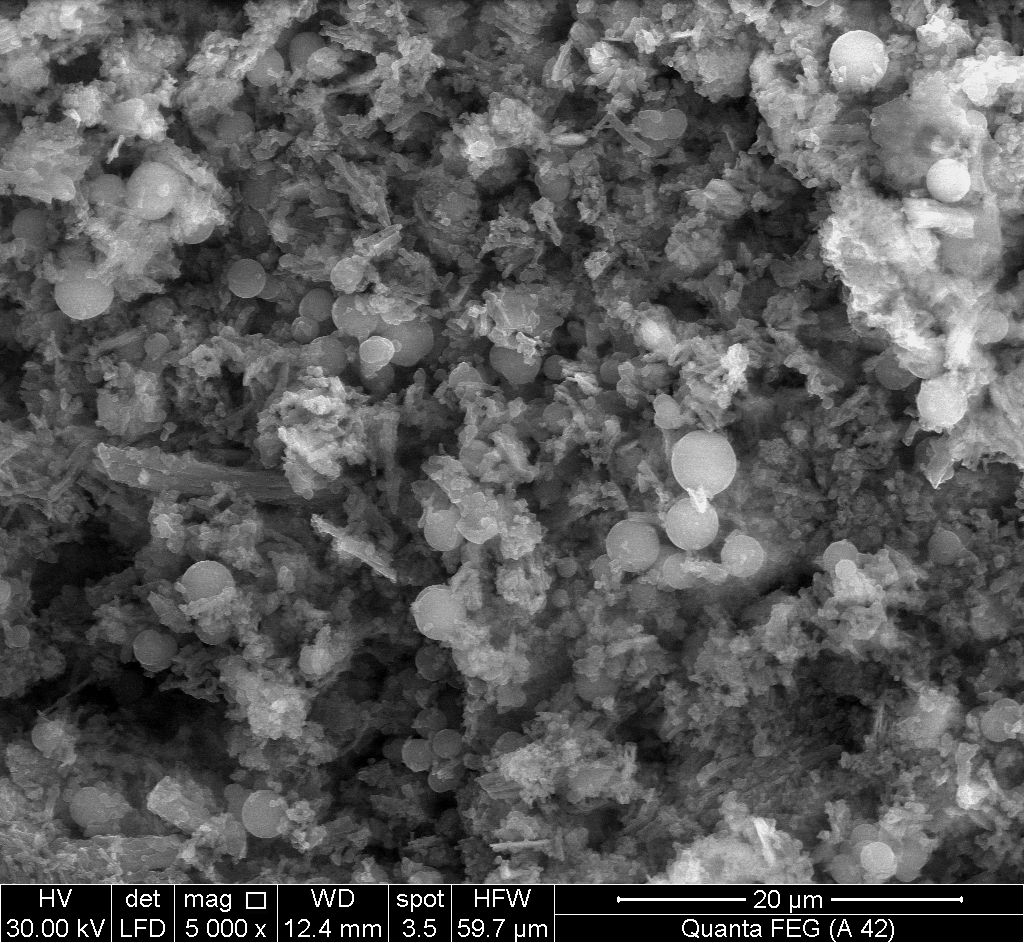 | Figure 17. SEM morphology image of (nZrP-nCePf)/polyindole-co-polypyrrole nanocomposite |
3.15.2. FTIR of (nZrP-nCePf) /Polyindol-Co-Polyaniline,/ Polyindole-Co-Polypyrrole Nanocomposite Membrane
- FTIR Spectra of the copolymers are shown in Figures (18, 19). A broad band centered at 3400 cm-1, due to the characteristic stretching vibration OH groups symmetric of H2O, superimposed with that of N-H stretching of aromatic amines. Small bands at the range 2960-2865cm-1 could be attributed to N-H stretching. Bands in the region 1680-1600cm-1 are related to stretching C-C bonds characteristic of indole unites C-H (aromatic) stretching, C=C stretching. C-N stretching (between two indole units), are in the region 1570-1370 cm-1 [9, 35-39]. The presence of bands in the range 1455–1373 cm−1 is assigned to the non-symmetric C6 ring stretching modes and contribution from the quinoid rings, the presence of benzenoid units of polyaniline Figure 18. However, the characteristic band for polypyrrole are found in the range of 1487-1176cm-1, Figure 19. Small sharp band at 1625 cm-1 is related to H-O-H bending, and sharp broad band centered at 1040 cm-1 is corresponds to phosphate groups vibration [33, 39].
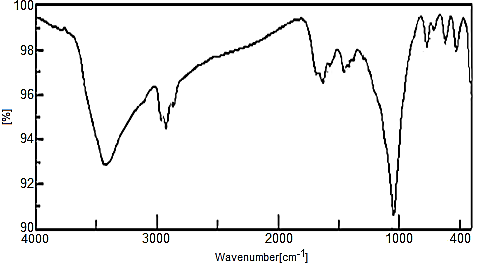 | Figure 18. FT-IR of (nZrP-nCePf)/polyindole-co-polyaniline nanocomposite |
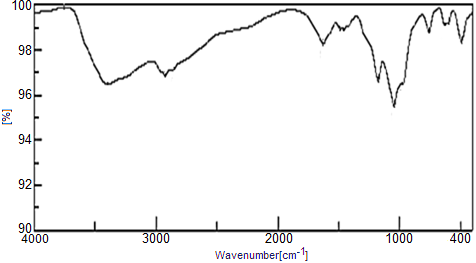 | Figure 19. FT-IR of nZrP-nCePf)/polyindole-co-polypyrrole nanocomposite |
4. Conclusions
- Exfoliated naosized zirconium phosphate, Zr(HPO4)2. H2O(nZrP), naosized fibrous cerium phosphate Ce(HPO4)2. 2.9H2O(nCePf) and their composite [Zr(HPO4)2]0.25 [Ce(HPO4)2]0.75.3.87H2O were prepared and characterized. Novel Zirconium phosphate-fibrous cerium phosphate/ polyindole,- / polyindole-co-polyaniline and -/polyindole -co-polypyrrole nanocompsite membranes were prepared via in-situ chemical oxidation of the indole, and its co-monomers, that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix of (nZrP-nCePf ). The presence of Ce(iv) ions allows redox reactions necessary to oxidative polymerization to occur. A possible explanation is that polymerization of indole and its copolymers was promoted by the reduction of some of nCePf present in (nZrP-nCePf) composite, that attacked by indole and its co-monomers, converted to cerium(III) orthophosphate (CePO4). The formulation of the resultant composites was supported by thermal, elemental (C,H,N) analysis, FT-IR spectrum and SEM. The amount of organic material present in (nZrP-nCePf)/PIn composites found to be (8.83% in wt.), where the amount of organic material present in (nZrP-nCePf)/ PIn-co-PANI found to be PIn = 17.51 PANI = 26.26 % in wt., and for (nZrP-CePf) /PIn-co-PPy found to be PIn = 21.84, PPy = 29.13 % in wt. It has been observed experimentally that copolymerization of two different monomers increases the number of conductive polymers which cannot be achieved by using single monomer The color of the resultant composites (were green, and blackish green), respectively, which may be due to the self doping occurred on polymerization, due to of H+ of (=POH) groups and slurry solution of (nCeP) [41]. The % in wt of each co-polymers from CHN analysis found to be in agreement with the % in wt contents of copolymer that recovered from HF solution experiment. Beneficial properties of both zirconium phosphate-fibrous cerium phosphate composite, zirconium phosphate-fibrous cerium phosphate /polyindole, -/polyindole-co-polyaniline and -/polyindole-co-polypyrrole nanocompsites can be considered these composites as novel conducting inorganic-organic composites, ion exchangers, solid acid catalyst and as sensors [6, 10, 39-41].
AKNOWLEDGEMENTS
- To Department of Chemistry, Faculty of Science, Tripoli University for the supporting of this research. To geologist Adel Bayuomi, mineral resources Centre, Egypt, for providing facilities for SEM, elemental (C,H,N) analysis and FT-IR spectroscopy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML