| [1] | Street, G. B., Clarke, T. C., Conducting Polymers: A Review of Recent Work IBM J. Res. Dev. 25(1981)51–57. |
| [2] | T. J. Skotheim, J. R. Reynolds, Eds. Handbook of Conducting Polymers, Third Ed., CRC Press, Boca Raton, USA (1997). |
| [3] | H. S. Nalwa, Ed. Handbook of Organic Conductive Molecules and Polymers, Vol. 2, John Wiley, Chichester, UK (1997). |
| [4] | MacDiarmid, A. G., A novel role for organic polymers , Angewandte Chemie International Edition, 40 (2001)2581. |
| [5] | Heeger, A., Semiconducting and metallic polymers: The fourth generation of polymeric materials, Reviews of Modern Phys , 73(2001)681. |
| [6] | Freund, M.S. and Deore, B.A. Edts., Self Doped Conducting Polymers, John Wiley and Sons (2007). |
| [7] | Lange, U., Roznyatovskaya, N. V., Mirsky, Vladimir M., Conducting polymers in chemical sensors and arrays, Analytica Chimica Acta vol. 614 issue (2008) |
| [8] | Kucheldorf, H.R., Luken, O. and Swift, G., Hand book of polymer synthesis, 2nd Edit., CRC Press (2010). |
| [9] | Banerjee, S. and Tayagi, A.K. Edts, Functional Materials: Preparation, Proscessing and Applications, Elsevier (2012). |
| [10] | Lange, U., Roznyatovskaya, N. V., Mirsky, Vladimir M., Conducting polymers in chemical sensors and arrays, Analytica Chimica Acta vol. 614 issue (2008) . |
| [11] | Sapurina, I. Y. and Shishov, M. A., New polymers for special applications, Ailton De Souza Gomes, Ed. (2012). |
| [12] | Cai, Z., Grang, M. and Tang, Z., Novel battery using conducting polymers: and polyaniline , J .of Mater. Sci., 39 (2004) 4001. |
| [13] | Ćirić-Marjanović, G., Polyaniline nanostructures, in nanostructured conductive polymers, (Eftekhari, A. ed., John Wiley & Sons, Ltd(2010). |
| [14] | Vernitskaya, T.V. Tat'yana, V.V. and Efimov, Polypyrrole: a conducting polymer; its synthesis, properties and applications, Russ. Chem. Rev., (1997)66 443. |
| [15] | Feng-Hao Hsu, F.H and, Wu, T.M., In situ synthesis and characterization of conductive polypyrrole/graphene composites with improved solubility and conductivity, Synthetic Metals, 162(2012) 682–687. |
| [16] | Fajan, A. and Been, B., structural and optical properties of polyindole manganese oxide nanocomposite, Indian J. of Adv. in Chem. Sci., 2 (2013) 95-96. |
| [17] | Shukla, S.K., Bharadvaja, A., Tiwari, A., Parashar, G.K.., and Dubey, G.C., Synthesis and characterization of highly crystalline polyaniline film promising for humid sensor., Adv.Mat.Lett., 1(2010) 129-134. |
| [18] | Chiang, J.C., MacDiarmid, A. G., Protonic acid doping of the emeraldine form to the metallic regime, Synthetic Metals , 1(1986) 193. |
| [19] | Ito, S., K. Murata, K., S. Teshima, S., R. Aizawa, R., Y. Asako, Y, Takahash K. and Hofmann, B. M., Simple synthesis of water-soluble conducting polyaniline, Synthetic Metals, 96(1998) 161. |
| [20] | Colak, N. and Sokman, B., Doping of Chemically synthesized polyaniline, Des Monomer Polym., 3 (2000) 181-189. |
| [21] | MacDiarmid, Alan G, A novel role for organic polymers , Angewandte Chemie International Edition, 40 (2001)2581. |
| [22] | Heeger, A., Semiconducting and metallic polymers: The fourth generation of polymeric materials, Reviews of Modern Phys , 73(2001)681. |
| [23] | Virji, S., H., Jiaxing; Kaner, R. B.; Weiller, B. H., Polyaniline nanofiber gas Sensors: examination of response mechanisms, Nano Letters, 4(2004) 491. |
| [24] | Ansaci, R.M. and Kervani, F.V., Polyaniline conducting electroactive polymers: thermal and environmental stability studies, J.Chem., 3(2006) 202-217. |
| [25] | Li, X., Oxidative polymerization of aniline using NaClO2 as an oxidant, Mater. Lett., 61,(2007) 2011. |
| [26] | Wessling, B., New insight into organic metal polyaniline morphology and structure, Polym., 2(2010) 786. |
| [27] | Sapurina, I. Y. and Shishov, M. A. , New polymers for special applications, Ailton De Souza Gomes ,Ed. (2012). |
| [28] | Chen.y, Li,Y., Yip, M., Taid, N., Electromagnetic interference shielding efficiency of polyaniline composites filled with graphene decorated with metallic nanoparticles ,J. Composite Sci.Tech,80(2013)80. |
| [29] | Sharma, D., Kaith, B.S. and Rajput, J, Single step in situ synthesis and optical properties of polyaniline/ZnO nanocomposites, The Sci. World J, Volume 2014 (2014), Article ID 904513, 13 pages. |
| [30] | Pandiselvi, K., Manikumar, A. and Thambidurai, S., Synthesis of novel polyaniline/MgO composite for enhanced adsorption of reactive dye, Polymer Composites, 35(2014)351. |
| [31] | Clearfield, A., Inorganic Ion Exchange Materials, Bocca Raton, CRC Press Fl.. (1982). |
| [32] | Colon, J.L., Diaz, A. and Clearfeild, A., Nanoincapsulation of insulin into zirconium phosphate for oral delivery applications, Biomacro Molecules, 9 (2010) 2465. |
| [33] | Diaz, A., Saxena,V., Gunzalez, J., David, A., Casanas, B., Carpenter, C., Batteas, J.D., Colon, J., Clearfield, A. and Hussain, M.D., Zirconium phosphate nano-platelets: a novel platform for drug delivery in cancer therapy, Chem. Commun., 48 (2012) 1754. |
| [34] | Tushato, M., Danjo, M., Baba,Y., Murakom, M. and Nana, H., Preparation and chemical properties of a novel layered cerium(iv) phosphate, Bulletin of Chem.Soc Jap., 70 ( 1997) 143. |
| [35] | Salvado, M.A., Pertierra, P., Tropajo, C. and Garcia, G.R., Crystal structure of cerium(iv) bis(hydrogen phosphate derivative, J. Am. Chem. Soc., 129)2007(10970. |
| [36] | Alberti, G. and Costantino, U. , Recent progress in the field of synthetic inorganic exchanger having a layered and fibrous structure, J. Chromatogr., 102 (1974)5. |
| [37] | Parangi, T., Wani, P. and Chudasama, U., Synthesis, characterization and application of cerium phosphate, Desalination and Water Treatment, 38 (2012)126. |
| [38] | Romano, R. and Alves, O.S., Fibrous cerium(iv) phosphate host of weak and strong Lewis bases, Inclus. Phenom. and Macrocyclic, 51 (2005) 211. |
| [39] | Casciola, M., Costantino, U. and D´amico, S., ac Conductivity of cerium(iv) phosphate in hydrogen form, Solid State Ionics, 28 (1988) 617. |
| [40] | Varsheny, K.G., Tayal, N., Gupta, P, Agrawal, A and Drabik, M., Synthesis, ion-exchange and physical studies on polystyrene cerium(iv) phosphate hybrid fibrous ion exchanger , Ind. J. of Chem. 43 (2004) 2586. |
| [41] | Metwally, S.S., El-Gammal, B., Ali, H.F. and Abo-EL-Enein, S.A., Removal and separation of some radionuclides by poly-acrylamide based Ce(iv) phosphate, Separation Sci. and Tech., 46 (2011)11. |
| [42] | Alberti, G., Casciola, M., Captani, D., Donnadio, A., Narducci, R., Pica, M. and Sganappa, M.,N., Novel nafion-zirconium phosphate composite membranes with enhanced stability of proton conductivity, Electrochemica Acta, 52 (2007)8125. |
| [43] | Yang, Y., Liu, C. and Wen, H., Preparation and properties of polyvinyl alcohol / exfoliated α-zirconium phosphate, Polym. Test. , 28 (2009)185. |
| [44] | Nagarale, R.K., Shin, W and Singh, P.K., Progress in ionic organic-inorganic composite membranes for fuel cell application, Polym. Chem., 1 (2010) 388. |
| [45] | Shakshooki, S.K, Elejmi, A. A., Khalfulla, A. M. And. Elfituri. S. S., Int. Conf. on Mater. Imperative, pp49-70 (CD). Cairo, Egypt , 29/11-2/12 (2010) . |
| [46] | Osaka, Y. and Nakagawa,T., Edt(r): Membrane science and technology, Marcel Dekker Inc N.Y., 1992. |
| [47] | Palacio, L., Ho, C., Paradanos, P., Calvo, J.I., Kherif, G., Larbot, A. and Hernandez, A.: Structure and charges of composite inorganic microfiltration membranes, J.Coll. and Surf., 138, 291299, 1998. |
| [48] | Strathmann, H., Giorno, L. and Drioli, E., Edt(r): An introduction to membrane science and technology, Consiglio Nationale Della Ricerche, Roma, 2006. |
| [49] | Oyama. S.T. and Williams. S.M..S, Edt(r): Inorganic polymeric and composite membranes, Elsevier vol.14, 2011 |
| [50] | Feng, Y., He, W., Zhang, X., Jia, X. and Zhao, H., The preparation of nanoparticle zirconium phosphate, Mater.. Letters, 61 (2007) 3258. |
| [51] | Bao, C., Gua, Y., Song, L., Lu, H., Yuan, B., and Hu, Y., Facile synthesis of poly(vinyl alcohol)/α-titanium phosphate nanocomposite with markedly enhanced properties, Ind. Eng. Chem. Res., 50 (2011)11109. |
| [52] | Verissimo, C. and Alves, O.L, Preparation of the conducting nanocomposites using molded inorganic matrix: fibrous cerium phosphate as a self supported pyrrole polymerization, J. Mater .Sci., 13 (2003)1378-1383. |
| [53] | Salomaki, M., Rasanan, M., Leiro, J., Huti, T., Tenho, M., Lukkari, J. and Kankari, J., Oxidative inorganic multilayer for polypyrrole film generation, Adv. Funct. Mater., 20 (2010) 2140-2147. |
| [54] | Rosenthal, G.L., Caruso, J., and Stone, S.G., Polymerization of aniline on copper zirconium phosphate and mixed phosphonate, Polyhedron, 13(1999)1311-1314. |
| [55] | Goncalves, A.B., Mangrich, A.S. and Zarbin, A.J.G., Polymerization of pyrrole between the layers of tin(iv) bishydrogen phosphate, Synthetic metals, 114(2000)119-124. |
| [56] | Oyama. S.T. and Williams. S.M..S, Edt(r), Inorganic polymeric and composite membranes, Elsevier vol.14, 2011. |
| [57] | Shakchooki, S.K., El-Akari, F.A., El-Fituri, M.S. and El-Fituri, S.S., Fibrous cerium(iv) hydrogen phosphate membrane self supported benzimidazole polymerization agent, Adv. Mater. Res., 856 (2014) 3. |
| [58] | Shakshooki, S.K., Nano Fibrous cerium (IV) hydrogen phosphate membrane self supported indole polymerization agent, J.Chem. Chem. Eng., 8(2014) 378-384. |
| [59] | Alberti. G, Bernasconi. M.G and Casciola. M, Preparation of γ-Zirconium phosphate microcrystals with light degree of crystallinity, Reactive Polymers, 11(1989), 245-252. |
| [60] | Parveen, A. and Roy, A., Effect of morphology on thermal stability of core-shell polyaniline/TiO2, Adv.Mat.Lett., 4(2013) 696-70. |
| [61] | Khuspe, G.D., Navale, S.T., Bandgar, Sakhare, D.K., Chugle, M.A., and Patil, V.B., SnO2 nanoparticles-modified Polyaniline Films as Highly Selective, Sensitive, Reproducible and Stable Ammonia Sensors, Electron. Mater. Lett., 10, (2014)191-197. |
| [62] | Jain R., Tiwari D.C .and Shrivastan S., Sensitive voltmmetric sensor based on synergistic effect of polyaniline and zirconia nanocomposite film, J. of Elect. Chem.Soci., 161(2014) B38-B44. |
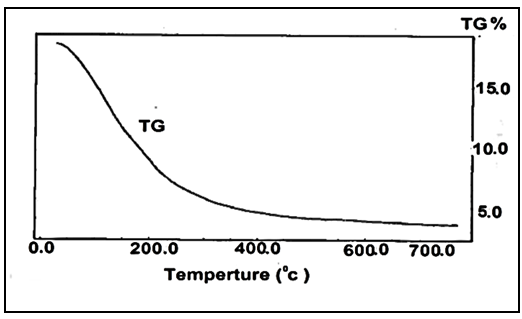
 -zirconium phosphate (γ-ZrP) in 150 ml distilled water was added gradually to slurry aqueous solution of 0.6g fibrous cerium in 450ml distilled water at 45°C, with stirring, the stirring was continued after complete addition and at 45°C for 24h. The resultant product was filtered on filter paper using Buchner funnel, washed with distilled water (50ml) twice then left to dry in air, the resulted product was homogeneous thin film, good mechanical texture, [γ-Zr.PO4.H2PO4.]0.34 [Ce(HPO4)2]0.66. 4.4H2 O was obtained.In similar manner, using the above experimental parameters, composite -zirconium phosphate-fibrous cerium phosphate membrane [γ-Zr.PO4.H2PO4]0.27 [Ce(HPO4)2]0.73.3.47H2O (γ-ZrP0.27-CePf0.73) was obtained by mixing slurry aqueous solution in wt/wt % ratio 25:75 of γ-ZrP:nCePf, where the mixing weight ratio is 0.2g:0.6g, respectively. After complete mixing of the above slurry aqueous solutions, at 45°C for 24h, the resultant product was filtered on filter paper using Buchner funnel, washed with distilled water and left to dry in air. 2.6. Preparation of γ-zirconium Phosphate-Fibrous Cerium Phosphate/Emeraldine Salt Nanocomposite Membranes γ-zirconium phosphate-fibrous cerium phosphate /emeraldine salt nanocompsite membranes were prepared by different methods:(1) By immersion of self supported-sheet (γ-ZrP0.34- CePf0.66), 150 mg in 15 ml of 4% aniline in ethanol at (~5°C), for 10 minutes, then kept at room temperature for 72h. The color change gradually from blue to dark green. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air, designated as composite (I). (2) By immersion of self supported-sheet (γ-ZrP0.27- CePf0.73), 150 mg in 15 ml of 4% aniline in ethanol at (~5°C), for 10 minutes, then kept at room temperature for 72h. The color changes gradually from blue to dark green. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air, designated as composite (II).(3) By immersion of self supported-sheet (γ-ZrP0.34- CePf0.66), 180 mg in 17.5 ml of 4% aniline in 1MHCl solution at room temperature for 72h. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air. The color of the resulting product was green, designated as composite (III).(4) By immersion of self supported-sheet (γ-ZrP0.27- CePf0.73), 180 mg in 17.5 ml of 4% aniline in 1MHCl solution at room temperature for 72h. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air. The color of the resulting product was green, designated as composite (IV).
-zirconium phosphate (γ-ZrP) in 150 ml distilled water was added gradually to slurry aqueous solution of 0.6g fibrous cerium in 450ml distilled water at 45°C, with stirring, the stirring was continued after complete addition and at 45°C for 24h. The resultant product was filtered on filter paper using Buchner funnel, washed with distilled water (50ml) twice then left to dry in air, the resulted product was homogeneous thin film, good mechanical texture, [γ-Zr.PO4.H2PO4.]0.34 [Ce(HPO4)2]0.66. 4.4H2 O was obtained.In similar manner, using the above experimental parameters, composite -zirconium phosphate-fibrous cerium phosphate membrane [γ-Zr.PO4.H2PO4]0.27 [Ce(HPO4)2]0.73.3.47H2O (γ-ZrP0.27-CePf0.73) was obtained by mixing slurry aqueous solution in wt/wt % ratio 25:75 of γ-ZrP:nCePf, where the mixing weight ratio is 0.2g:0.6g, respectively. After complete mixing of the above slurry aqueous solutions, at 45°C for 24h, the resultant product was filtered on filter paper using Buchner funnel, washed with distilled water and left to dry in air. 2.6. Preparation of γ-zirconium Phosphate-Fibrous Cerium Phosphate/Emeraldine Salt Nanocomposite Membranes γ-zirconium phosphate-fibrous cerium phosphate /emeraldine salt nanocompsite membranes were prepared by different methods:(1) By immersion of self supported-sheet (γ-ZrP0.34- CePf0.66), 150 mg in 15 ml of 4% aniline in ethanol at (~5°C), for 10 minutes, then kept at room temperature for 72h. The color change gradually from blue to dark green. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air, designated as composite (I). (2) By immersion of self supported-sheet (γ-ZrP0.27- CePf0.73), 150 mg in 15 ml of 4% aniline in ethanol at (~5°C), for 10 minutes, then kept at room temperature for 72h. The color changes gradually from blue to dark green. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air, designated as composite (II).(3) By immersion of self supported-sheet (γ-ZrP0.34- CePf0.66), 180 mg in 17.5 ml of 4% aniline in 1MHCl solution at room temperature for 72h. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air. The color of the resulting product was green, designated as composite (III).(4) By immersion of self supported-sheet (γ-ZrP0.27- CePf0.73), 180 mg in 17.5 ml of 4% aniline in 1MHCl solution at room temperature for 72h. The impregnated sheet was removed, washed with distilled water and ethanol and left to dry in air. The color of the resulting product was green, designated as composite (IV). 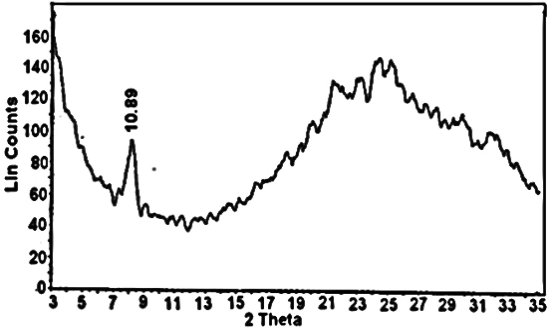
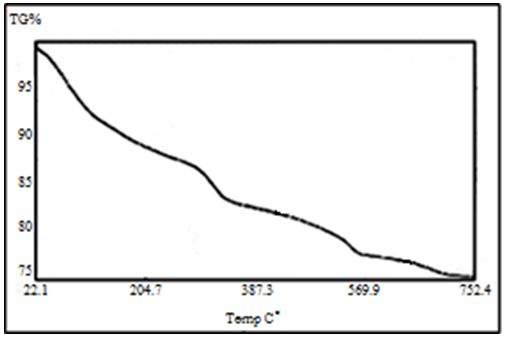
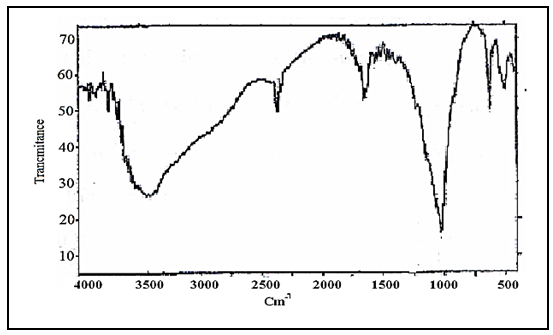
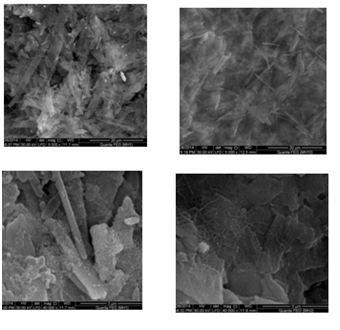
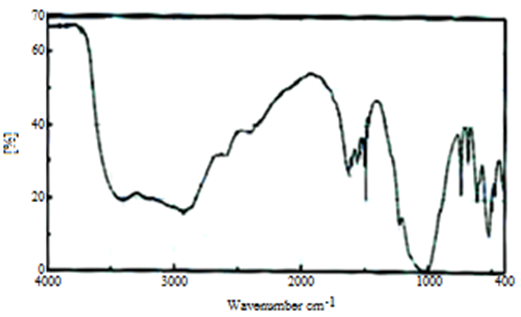
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML