-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(3): 67-74
doi:10.5923/j.chemistry.20150503.01
Nano Fibrous Cerium(IV) Hydrogen Phosphate Membrane Self Supported Aniline Polymerization Agent
S. K. Shakshooki, M. A. Abuain, Amani M. El-Aouzi
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: S. K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Nanosized fibrous cerium(iv) Hydrogen phosphate membrane, Ce(HPO4)22.9H2O (nCePf), was prepared and characterized. Novel supported nanofibrous cerium phosphate/ polyanaline nanocomposite membranes were prepared via in-situ chemical oxidation of the monomer aniline, that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix. The presence of Ce(iv) ions allows redox reactions necessary to oxidative polymerization to occur. The resultant materials were characterized by TGA, elemental (C, H, N, S) analysis and FT-IR spectroscopy. Images SEM of the resulting nanocomposites reveal a uniform distribution of the polymer on the inorganic matrix. A possible explanation is nCePf present on the surface of the fiber is attacked by the aniline and converted to cerium(III) orthophosphate(CePO4). The rest of the (nCePf) was preserved. The degree of polymer loading and its type can be controlled by varying method of the preparation. Accordingly two types of green emeraldine salts (in form of chloride and sulfate salts) were obtained. Amount of polyaniline present in the resultant composites found to be in the range of (4.5- 16.74% in weight).
Keywords: Nanofibrous cerium (iv) hydrogen phosphate membrane, Aniline polymerization
Cite this paper: S. K. Shakshooki, M. A. Abuain, Amani M. El-Aouzi, Nano Fibrous Cerium(IV) Hydrogen Phosphate Membrane Self Supported Aniline Polymerization Agent, American Journal of Chemistry, Vol. 5 No. 3, 2015, pp. 67-74. doi: 10.5923/j.chemistry.20150503.01.
Article Outline
1. Introduction
- Conducting polymers are a novel class of synthetic metals that combine the chemical, electrochemical and mechanical properties of polymers with the electronic properties of metals and semiconductor, generated tremendous interest due to their potential applications in various fields such as rechargeable batteries, electrochromic display devices, separation membranes, sensors and anticorrosive coatings on metals [1-11]. Heterocyclic conducting polymers containing nitrogen atoms like polyaniline, polyindole and polypyrrole, and their substituted derivatives have received increasing attention in various fields, electronics, industry and others. Their electrical and electrochemical properties show great promise for commercial applications. They provide an interesting and useful focus for expansion of the fields of polymeric reagent research and molecular engineering. They are electrically conductive which makes their use as metal replacement materials of some interest [12-16].The properties of electronically conductive polymers depend strongly on their microstructur and morphology, and these determined by the synthesis method, counter ion and several other variables [17].Polyaniline is one of the most studied conducting polymers, has potential for applications due to its light weight, conductivity, mechanical flexibility and low cost. Polyaniline is especially attractive because it is relatively inexpensive. It has certain advantages over other conducting polymers, including simplicity and rapidity of preparation by electrochemical and oxidative chemical polymerization methods, and the ability to be formed in aqueous electrolytic solutions. It has good environmental stability and its conductivity could be controlled with acid/base (doping/ undoping) [12, 13, 17-30].Inorganic layered nanomaterials are receiving great attention because of their size, structure, and possible biochemical applications [31, 32], that have been proven to be good carriers for organic polar molecules. Examples of these are zirconium phosphates. Taking advantage of the expandable interlayer space of the layered materials. Researchers have been capable of encapsulating functional biomolecules into these inorganic matrices protecting them from interacting with environment, avoiding denaturation and enhancing their shelf life [31, 32]. Crystalline cerium phosphates have been studied for a long time as ion exchangers, their structures remains unknown until recently [33-35]. The reason is that, the composition, the structure and the degree of crystallinity of their precipitates results from reaction of solutions containing a Ce(iv) salt is mixed with a solution of phosphoric acid of [(PO4)/ CeIV ratio], strongly dependent on the experimental conditions such as rate and order of mixing of the solutions, stirring, temperature and digestion time, this also implemented on fibrous cerium phosphate [36]. To date most of the work on fibrous cerium phosphate was carried out on its ion exchange [37], intercalation [38] and electrical conductance properties [39]. Studies on its polystyrene, polyacrylamide [40] and (polyvinyl chloride-based polyvinyl (alcohol) [41] composites have been reported. Nanoscaled tetravalent metal phosphates and their organic polymer composites comprise an important class of synthetic engineering. However; research in such area is still its infancy [42-45]. Nanotechnologies are at the center of numerous investigations and huge investments. However, chemistry has anticipated for long the importance decreasing the size in the search of new properties of materials, and of materials structured at the nanosize in a number of applications relate to daily life. Organic-inorganic nanocomposite membranes have gained great attention recently [45, 46], The composite material may combine the advantage of each material, for instance, flexibility, processability of polymers and the selectivity and thermal stability of the inorganic filler [43-47]. Pyrrole polymerization by fibrous cerium phosphate has been reported [48, 49]. polymerization of Pyrrole and aniline on α-Tin phosphate and copper zirconium phosphate was reported [50, 51]. In our laboratory we are carrying systematic investigations on novel tetravalent metal phosphates / organic heterocyclic conducting polymers nanocomposite membranes. Recently we have reported [52, 53] the preparation and characterization of fibrous cerium phosphate/ polybenzimidazole nanocomposite membrane and fibrous cerium phosphate/ polyindole nanocomposite membranes. The present study describes the preparation and characterization of novel supported fibrous cerium phosphate/ polyaniline nanocomposite membranes via in-situ chemical oxidation, of the monomer aniline, that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix.
2. Materials and Methods
2.1. Chemicals
- Ce(SO4)2.4H2O, H3PO4(85%) of BDH, aniline (99.5%) of Mindex UK. Other reagents used were of analytical grade.
2.2. Instruments Used for Characterization
- X-Ray powder diffractometer Siemens D-500, using Ni-filtered CuKα (λ= 1.54056Å), TG/DTA SIIExtra6000 TGA Perkin Elmer thermogravimetric analyzer(TGA7)US, CHNS-Elmental Analysis, Vario Elementa German. Fourier Transform IR spectrometer, model FT/IR-6100, Scanning electron microscopy (SEM) Jeol SMJ Sm 5610 LV.
2.3. Preparation of Nanofibrous Cerium Phosphate Membrane, Ce(HPO4)2.2.9H2O
- Nanofibrous cerium phosphate membrane was prepared from adding 300 ml of 0.05M CeSO4.4H2O in 0.5 M H2SO4 solution, drop wise, to 300 ml of 6 M H3PO4 at ~ 80C with stirring. After complete addition the resultant material left to digest at that temperature for 4h. To that 3 liter of hot distilled water, (~60C), was added with stirring for 1h. The resulting fibrous cerium phosphate was subjected to washing with distilled water up to pH 3, then filtered and left to dry in air.
2.4. Exchange Capacity
- Exchange capacity of the resultant nanosized fibrous cerium phosphate membrane was determined by addition of 25 ml of 0.1M NaCl solution to 100 mg of the material, with stirring for one h, then titrated with 0.1 M NaOH solution.
2.5. Thermal Analysis
- Thermal analyses were carried out at temperature range about 20 -775C in nitrogen atmosphere, the rate was 10C/min.
2.6. Preparation of Nanofibrous Cerium Phosphate Polyaniline Composite Membranes
- Cerium phosphate /polyaniline nanocompsite membranes were prepared by different methods:(1) By immersion of fibrous cerium phosphate self supported-sheet 160 mg in 10 ml ethanol solution containing 180 mg aniline in10 ml ethanol solution, at room temperature , for 48h. The impregnated sheet was removed, washed with ethanol and left to dry in air. The color of the resulting product was dark-blackish- blue, designated as composite(I).(2) By immersion of fibrous cerium phosphate self supported-sheet 160 mg in 10 ml ethanol solution containing 180 mg aniline at room temperature for 1h. The impregnated sheet was removed, washed with ethanol and left to dry in air. The color of the resulting product was pale-blue, designated as composite(II).(3) immersion of fibrous cerium phosphate self supported-sheet 160 mg in 10 ml 1MHCl solution for 24h, resulting impregnated sheet of pale green in color, it was washed with ethanol and left to dry in air, and designated as composite(III).(4) Addition of 0.5ml aniline to 35 ml of the original slurry aqueous solution of fibrous cerium phosphate (prior subjecting to washing, its pH =1.6), with stirring at room temperature leading to immediate formation of green polyaniline, (emeraldine salt). The stirring of the mixture was continued for an hour. The contents left in fridge (5C) for 24h. The product was filtered washed with ethanol and left to dry in air. The color of the resultant product dark green, designated as composite (IV).Nanofibrous cerium phosphate membrane, Ce (HPO4)2.2.9H2O (nCePf), was prepared and characterized by chemical, XRD, TGA, FT-IR, and SEM.1. XRDXRD of (nCePf) is shown in Figure 1, with d001 = 10.89Å.2. TGAThermogram of (nCePf) is shown in Figure 2. The thermal decomposition occurs in continuous process, the thermal analysis was carried out at temperatures between 10-775°C, the final product was CeP2O7. Loss of water of hydration occurs between 60-200°C, followed by POH groups condensation. The total weight loss found to be equal to 19.09%.
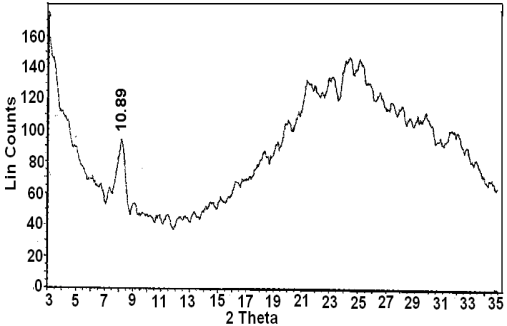 | Figure 1. XRD of (nCePf) |
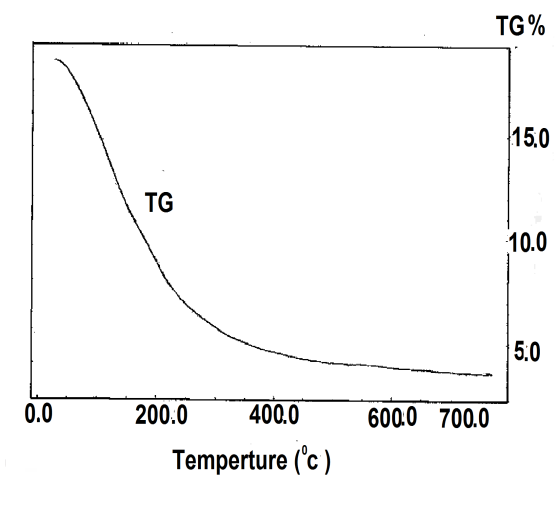 | Figure 2. TGA of (nCePf) |
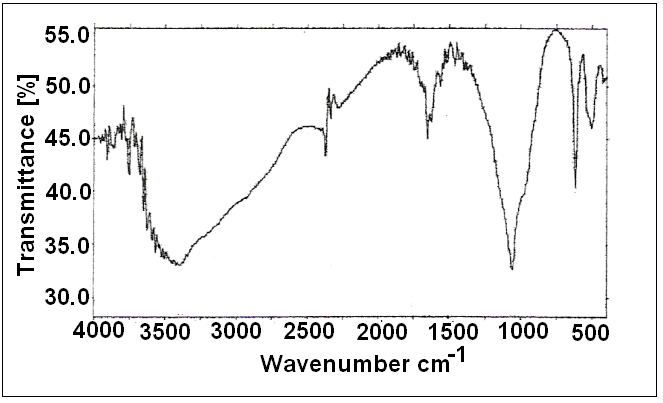 | Figure 3. FT-IR spectra of (nCePf) |
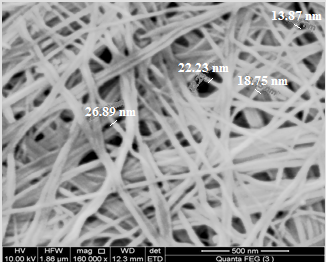 | Figure 4. SEM morphology image of (nCePf) |
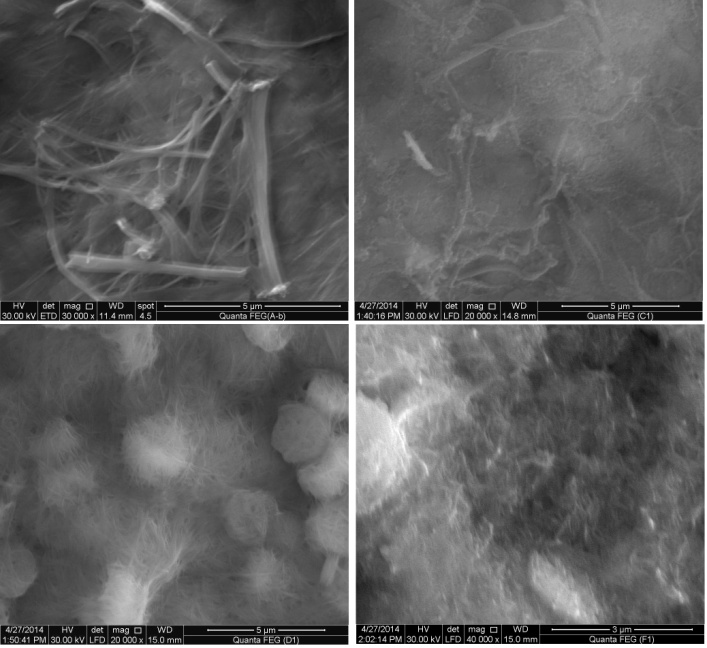 | Figure 5. SEM images of (nCePf)/PANI nanocomposites (I-IV) respectively |
- 7. SEMWe have noticed, from SEM analysis, the nanosized fibrous cerium phosphate morphology was preserve, consequently physical integrity of the resulting nanocomposites were maintained. Figure (5) shows scanning electron microscopy (SEM) images for (nCePf) / polyaniline nanocomposites (I), (II), (III) and (VI), respectively, reveal a uniform distribution of the polymer on the inorganic matrix, coating the nCePf and filling the empty spaces between the fibers. Inspite of the likely conversion some of nCePf to CePO4, nanocomposites present fibrous morphology. A possible explanation is nCePf present on the surface of the fiber is attacked by the aniline and converted to cerium (III) orthophosphate. The rest of the nCePf was preserved.8. FT-IR of NanocompositesFigure (6) shows FT-IR spectrum of (nCePf)/ polyaniline nanoocomposites (I, II III and IV ), respectively, (putting in consideration slight shifts of some bands as well as the low band intensity of composite (1) in the region 1723-1430cm-1) this was attributed to low loading of PANI in the composite). Broad band centered around ~3442 cm-1, is due to OH groups symmetric stretching of H2O super imposed with the N–H stretching of aromatic amines(expected at the range 3500-3280 cm-1). Very small band around ~1620 cm-1 is related to H-O-H bending , and sharp broad band, centered at ( ~1048 or ~1005 cm-1 ) corresponds to phosphate groups vibration. Small band at ~2924 cm-1 corresponds to C-H bonds. The presence of two bands in the range of 1490, 1303cm-1 is assigned to the non-symmetric C6 ring stretching modes. However the higher frequency vibration at ~1573 cm−1 has a major contribution from the quinoid rings (C=C stretching vibration of quinoid ring), while the lower frequency mode at ~1490 cm−1 depicts the presence of benzenoid rings (C=C stretching vibration of of benzenoid ring). Thus FTIR spectra confirms the formation of composites [54, 55]. 9. TGA of NanocompositesThermogram(TG/DTA) of (nCePf) / PANI , composite (I) , is shown in Figure(7). The weight loss up to 200°C (9.61%) is due to the removal of external water molecules. The weight loss occurs between 200-700°C corresponds to the decomposition of polyaniline and condensation of P-OH groups of the inorganic material to pyrophosphate (CeP2O7). The POH groups condensation found to superimpose with that of polyaniline decomposition. Thermal decomposition was accompanied with four endothermic peaks at 7o, 190, 300, 350°C. The total weight loss found to be equal to 21.899%. From thermal and elemental (C, H, N) analysis the resultant product was formulated as: Ce(HPO4)2 (PANI)x. 2.2 H2O, where x = 7.82%.
 | Figure 6. FT-IR of (nCePf)/PANI nanocomposites(I-IV) respectively |
- Thermogram (TG/DTA) of (nCePf) / PANI, composite (IV), is also shown in Figure (7), in respective manner. The weight loss up to 200°C (8.93%) is due to the removal of external water molecules. The weight loss occurs between 200-800°C corresponds to the decomposition of polyaniline and condensation of P-OH groups of the inorganic material to pyrophosphate (CeP2O7). The POH groups condensation found to superimpose with that of polyaniline decomposition. Thermal decomposition was accompanied with three endothermic peaks at 90, 240, 560°C. The total weight loss found to be equal to 32.203%. From thermal and elemental (C, H, N, S) analysis the resultant product was formulated as: Ce(HPO4)2 (PANI)x (SO42-)y .2.51 H2O, where x = 17.71% in weight, y=2.05 % in weight.
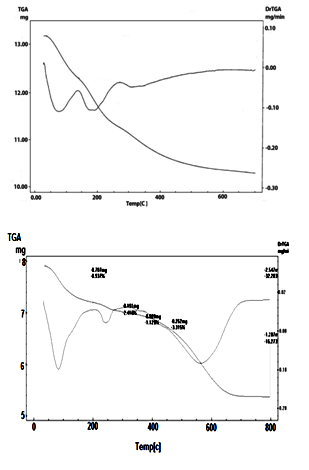 | Figure 7. TG/DTA of (nCePf)/polyailine composites(I & IV) respectively |
3. Conclusions
- Novel supported nanofibrous cerium phosphate/ polyaniline nanocomposite membranes were prepared via in-situ chemical oxidation of the monomer that was promoted by the reduction of Ce(iv) ions present in the inorganic matrix. The presence of Ce(iv) ions allows redox reactions necessary to oxidative polymerization to occur. The self-supported sheet integrity is preserved. This result is interesting because the shape integrity makes the building of molded conducting device possible. The formulation of the fibrous cerium phosphate nanocomposites was supported by thermal, elemental (C, H, N) analysis, FT-IR spectrum and SEM. The type of polyaniline composite and the degree of polymer loading found to be dependant on variation of method of the preparation and time aging. Polyaniline (PANI) is generally considered one of the most intriguing material for sensing devices due to its environmental stability, high conductivity. Thus by utilizing the beneficial properties of both cerium phosphate and PANI, their hybrid can be considered as novel conducting inorganic-organic composites, ion exchangers, solid acid catalyst and as sensors [10, 55, 56].
ACKNOWLEDGEMENTS
- To Department of Chemistry, Faculty of Science, Tripoli University, for providing facilities for this research, to Geol. Adel Bayuomi, Institute of Mineral Resources, Cairo, Egypt for providing facilities for elemental CHNS , FT-IR, TGA, SEM analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML