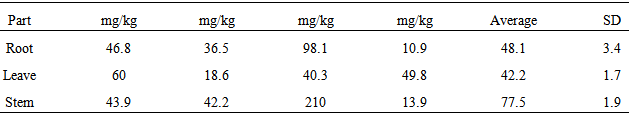
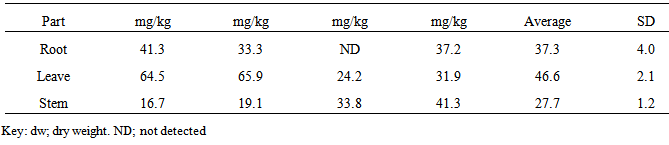
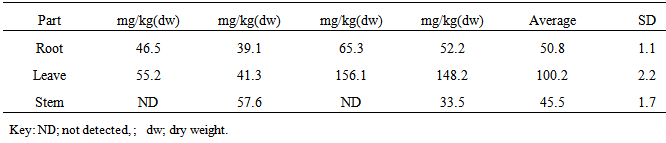
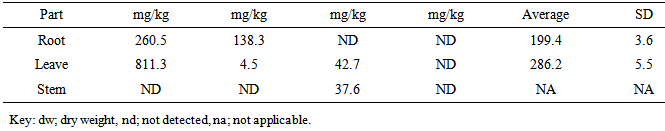
| [1] | Adewuyi, G.O., Okonkwo, J. and Okoli, C.P. (2009). Assessment of some heavy metals in soils and waterleaf (Talinum triangulare) in the vicinity of major quarry factory in Ibadan metropolis, Nigeria. Fresenius Environmental Bulletin 18(12):2396-2401. |
| [2] | Goel, S., Malik, J.A. and Nayyar, H. (2009). Molecular approach for phyto-remediation of metal contaminated sites. Archives of Agronomy and Soil Science 55(4):451-475. |
| [3] | Guala, S.D., Vega, F.A. and Covelo, E.F. (2010). Heavy metal concentration in plants and different harvestable parts; A soil-plant equilibrium model. Environmental Pollution 158:2659-2663. |
| [4] | Chehregani, A., Malayeri, B. and Golmohammadi, R. (2005). Effect of heavy metals on the developmental stages of ovules and embryonic sac in Euphorbia cheirandenia. Pakistan Journal of Biological Sciences 8:622-626. |
| [5] | Zahran, S., Mielke, H.W., Weiler, S., Hempel, L., Berry, K.J. and Gonzales, C.R. (2012). Associations between standardized school performance tests and mixtures of Pb, Zn, Cd, Ni, Mn, Cu, Cr, Co and V in community soil of New Orleans. Environmental Pollution 169: 128-135. |
| [6] | Sharmin, S. A., Alam, I., Kim, K., Kim, Y., Kim, P.J., Bahk, J.D. and Lee, B., (2012). Chromium-induced physiological and proteomic alterations in roots of Miscanthus sinensis. Plant Science 187;113-126. |
| [7] | Wierzbicka, M. and Obidzinska, J. (1998). The effect of lead on seed imbibition and germination in different plant species. Plant Science 137:155-171. |
| [8] | Dan, T., Hale, B., Johnson, D., Conard, B., Stiebel, B. and Veska, E. (2008). Toxicity threshold for oat (Avena Sativa I) grown in Ni-impacted agricultural soils near Port Colborne, Ontario Canada. Canadian Journal of Soil Science 88: 389-398. |
| [9] | McBride, M.B. (1995). Toxic metal accumulation from agricultural use of sludge are USEPA regulations protective? J. Environ. Qual. 24:5-18. |
| [10] | Frost, H.L. and Ketchum Jr, L.H. (2000). Trace metal concentration in durum wheat from application of sewage sludge and commercial fertilizer. Advances in Environmental Research 4:347-355. |
| [11] | Baldantoni, D., Alfani, A., Tommasi, P.D., Bartoli, G. and DeSanto, A.V. (2004). Assessment of macro and micro element accumulation capability of two aquatic plants. Environmental Pollution 130:149-156. |
| [12] | Osundiya M. O., Ayejuyo O. O., R. A. Olowu, Bamgboye O. A. and Ogunlola A. O (2014). Advances in Applied Science Research 5(1), 1-7 |
| [13] | McBride, M.B. (2007). Trace metals and sulfur in soil and forage of a chronic wasting disease locus. Environ. Chem 4;134-139. |
| [14] | Singh, B.R., Gupta, S.K., Azaizeh, H., Shilev, S., Sundre, D., Song, W.Y., Martinoia, E. and Mench, M. (2011). Safety of food crops on land contaminated with trace elements. J. Sci-Food Agric. 91: 1349-1366. |
| [15] | Wei, S., Zhou, Q. and Wang, X. (2005). Identification of weeds of plants excluding the uptake of heavy metal. Environmental International 31:829-834. |
| [16] | Neumann, D. and zur Nieden, U. (2001). Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685-692. |
| [17] | Schwartz, C., Gerard, E., Perronnet, J.K. and Morel, J.L. (2001). Measurement of Phytoextraction of zinc by spontaneous metallophytes growing in a former smelter site. Sci. Total Environ. 279:215-221. |
| [18] | Peralta-Videa, J.R., De la Rosa, G., Gonzalez, J.L. and Garrdea-Torresdey, J.H. (2004). Effect of growth stage on the heavy metal tolerance of alfalfa plants. Advances in Environmental Research 8:679-685. |
| [19] | Mattina, M.J.I., Lannucci-Berger, W., Eitzer, B.D. and White, J.C. (2004). Rhizotron study of cucurbitaceae: Transport of soil bound chlordane and heavy metal contaminants differ with genera. Environ. Chem.1:86-89. |
| [20] | Ince, N.J. (1999). Assessment of toxic interaction of heavy metals in binary mixture a statistical approach. Arch. Environ. Contam. Toxic. 36:365-372. |
| [21] | Gonzalez, R.C. and Gonzalez-chavez, M.C.A. (2006). Metal accumulation in wild plants surrounding mining wastes. Environmental Pollution 144:84-92. |
| [22] | Kabata - Pendias, A. and Pendias, H., 2001. Trace Elements in Soils and Plants, 3rd Edition, CRC Press, Boca Raton, London, 413 p. |
| [23] | Fischerova, Z., Tlustos, P., Szakova, J. and Sichorova, K. (2006). A comparison of phytoremediation capability of selected plant species for given trace elements. Environmental Pollution 144:93-100. |
| [24] | Wenzel, W.W., Bunkowski, M., Puschenrerter, M. and Horak, O. (2003). Rhizosphere characteristics of indigenous growing nickel hyperaccumulation and excluder plant on serpentine soil. Environmental Pollution 123:131-138. |
| [25] | Poschenrieder, C., Bech, J., Llugany, M., Peace, A., Fences, E. and Barcelo, J. (2001). Copper in plant species in copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230: 247-256. |
| [26] | Kamal, M., Ghaly, A.E., Mahmoud, N. and Cote, R. (2004). Phytoaccumulation of heavy metals by aquatic plants. Environmental International 29:1029-1039. |
| [27] | Kopittke, P.M., Asher, C.J., Blamey, F.P.C. and Menzies, N.W. (2008). Tolerance of two perennial grass to toxic level of Ni2+. Environmental Chemistry 5:426-434. |
| [28] | Murray, P., Ge, Y. and Hendershot, W.H. (2000). Evaluating three trace metal contaminated sites: a field and laboratory investigation. Environmental Pollution 107;127-135. |
| [29] | Santa-Maria, G.E. and Cogliatti, D.H. (1998). The regulation of zinc uptake in wheat plants. Plant Science 137;1-12. |
| [30] | Deng, H., Ye, Z.H. and Wong, M.H. (2004). Accumulation of lead, zinc, copper, and cadmium by 12 wetland plant species thriving in metal-contaminated sites in china. Environmental Pollution 132:29-40. |
| [31] | McBride, M.B. (2003). Toxic metal in sewage sludge amended soils has promotion of beneficial use dissented the risk? Advances in Environment Research 8:5-19. |
| [32] | Soltan, M.E. and Rashed, M.N. (2003). Laboratory study on the survival of water hyacinth under severe condition of heavy metal concentration. Advances in Environmental Research 7:321-334. |
| [33] | Nabulo, G., Black, C.R. and Young, S.D. (2011). Trace metal uptake by tropical vegetable grown on soil amended with urban sewage sludge. Environmental Pollution 159: 368-376. |
| [34] | Mohammad, A., Moheman, A. and Seema, A. (2009). The influence of a single and multiple soil contamination of cadmium with lead and zinc on growth, chlorophyll content, uptake and translocation of cadmium in tomato plants. Archives of Agronomy and Soil Science 55(4):407-413. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML