-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2015; 5(1): 23-27
doi:10.5923/j.chemistry.20150501.04
Antioxidant Activity and Total Flavonoids Content of Aerial Parts of Ficus pyriformis Hook. & Arn. (Moraceae) Cultivated in Egypt
Zedan Zeid Ibraheim1, Alaa Mohammed Nafady2, Mahmoud Abdullah Mostafa2, Fahd Mohammed Amin2
1Department of Pharmacognosy, Faculty of Pharmacy, Assuit University, Assuit, Egypt
2Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Assuit, Egypt
Correspondence to: Fahd Mohammed Amin, Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Assuit, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The total methanolic extract and fractions of Ficus pyriformis at different concentrations were subjected to free radical scavenging activity using 2,2-diphenyl 1-picrylhydrazyl (DPPH•) method. Different fractions of Ficus pyriformis obtained from successive fractionation of the total extracts on vacuum liquid chromatography (VLC) with organic solvents of different polarities. n-hexane, dichloromethane (DCM), ethyl acetate (EtOAc) and methanol (MeOH); showed that, the MeOH and EtOAc fractions have the highest activity followed by DCM and n- hexane fractions respectively. The total flavonoids content were measured using aluminum chloride colorimetric method and quercetin [QE] as standard equivalent for comparison, the total extract of Ficus pyriformis was also subjected to preliminary phytochemical screening tests for different phytoconstituents present in the plant.
Keywords: Antioxidant, DPPH• assay, Phytochemical screening, Ficus pyriformis
Cite this paper: Zedan Zeid Ibraheim, Alaa Mohammed Nafady, Mahmoud Abdullah Mostafa, Fahd Mohammed Amin, Antioxidant Activity and Total Flavonoids Content of Aerial Parts of Ficus pyriformis Hook. & Arn. (Moraceae) Cultivated in Egypt, American Journal of Chemistry, Vol. 5 No. 1, 2015, pp. 23-27. doi: 10.5923/j.chemistry.20150501.04.
Article Outline
1. Introduction
- Free radicals are molecules or molecular fragments containing one or more unpaired electrons in its outermost atomic or molecular orbital and are capable of independent existence and are involved in the normal physiology of living organisms [1, 2]. Under certain conditions, the excess of free radicals and Reactive Oxygen Species (ROS) like peroxy radical (ROO•) have been proposed to induce cellular damage and to be involved in several human diseases such as cancer, arteriosclerosis, inflammatory disorders as well as in ageing process [3]. Antioxidants are chemical substances that reduce or prevent oxidation. They have the ability to counteract the damaging effects of free radicals in tissues and thus are believed to protect against several diseases [4]. Therefore there is great interest in finding new and safe antioxidants from natural sources [5]. The genus Ficus have shown diverse biological activity, they have been investigated as potential repository of natural products for treatment of various ailments including tumors, inflammatory disorder, diabetes and as antioxidants [6, 7]. Flavonoids considered as one of the major classes of phytoconstituents in plants responsible for antioxidant activity and many Ficus species rich in flavonoids [8, 9]. The literature survey show that there is no report considering antioxidant activity of Ficus pyriformis. In this study we aimed to evaluate the antioxidant activity of Ficus pyriformis cultivated in Egypt, estimate the total flavonoids content and carry out preliminary phytochemical screening.
2. Material and Methods
2.1. Plant Material
- The aerial parts of Ficus pyriformis were collected during the flowering and fruiting stage from El-Orman Botanical Garden, Giza, Egypt. The specimens were authenticated by Ms. Trease Labib Consultant of plant taxonomy at the Ministry of Agriculture.
2.2. Preparation of Plant Extracts
- The plant material was collected, dried in shade, powdered, sieved and kept in an amberd well closed container in dark. The air-dried powdered aerial parts (2 Kg) of Ficus pyriformis. Were extracted by maceration and percolation with (70%) methanol three times and pooled together. The combined methanolic extract was concentrated under reduced pressure (40℃) using a rotary evaporator till constant weight to give a dark brown syrupy residue (150g). A part of the methanolic extract (100g) was subjected to successive solvent fractionation on VLC with n-hexane, DCM, EtOAc and finally with MeOH till complete exhaustion. Each fraction was filtered through filter paper Whatman no.1 and then concentrated under pressure (40℃) using rotary evaporator yielding respectively n-hexane (13g), DCM (11g), EtOAc (21 g) and MeOH (44 g) all kept in refrigerator till used.
2.3. Apparatus
- All spectroscopic data were acquired using the Shimadzu 1601, UV/visible Spectrophotometer. Disposable cuvettes (1 cm × l cm x 4.5 cm) were used for visible absorbance measurements. Rotary Evaporator 4000 (Heidolph, Germany).
2.4. Chemicals
- 2, 2-Diphenyl-1-picryl hydrazyl (DPPH•), quercetin (QE), ascorbic acid and potassium acetate were obtained from Sigma-Aldrich Chemicals Co., Germany. Aluminum chloride obtained from El-Nasr Pharmaceutical and Chemical Co., Egypt (ADWIC). Other chemicals used were of high analytical grade and obtained from Merck Chemical Co., Germany.
2.4.1. Determination of Total Flavonoids
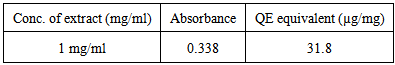
- Aluminum chloride colorimetric method was used for estimation of total flavonoids [10, 11]. 0.5 ml solution of plant extract was mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. The mixture kept at room temperature for 30 min. The absorbance of the reaction mixture measured at λ max 415 nm. Standard calibration curve is generated by using quercetin as reference standard. Stock solution of quercetin was made by dissolving 10 mg in methanol and transferred to volumetric flask and completes the volume to 10 ml, then makes serial dilution to make concentrations (10-100 μg/ml) in methanol.
2.4.2. DPPH Radical Scavenging Activity (DPPH• assay)
- The method of [12, 13] was adapted for testing the radical scavenging of the extracts using the stable free radical 2, 2-diphenyl-1-picrahydrazy (DPPH•) spectrophotometry. 10 × 10-5 M solution of DPPH• (394.3 g/mol.) was prepared by dissolving 0.04 g of DPPH• in 1000 ml of methanol. In the assay 0.2 ml of methanol solution of Ficus pyriformis aerial parts total extract and its fraction at different concentrations (0.0625,0.125, 0.250, 0.5, 1mg/ml) was mixed with 2 ml of methanol solution of DPPH• (0.1mM). Similarly; 0.2 ml methanol solution of ascorbic acid and quercetin of various concentrations (0.0625, 0.125, 0.25, 0.5, 1 mg/ml) were mixed with 2 ml of DPPH• solution. A mixture of 0.2 ml of methanol and 2 ml of methanol solution of DPPH• (0.1 mM) served as control. After mixing, all the solutions were incubated in dark for 30 min. and then absorbance was measured at λ max 517 nm. The experiments were carried out in triplicate using ascorbic acid and quercetin as a reference standards and DPPH• radical scavenging activity was calculated by using the formula [14].
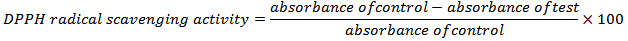
2.4.3. Phytochemical Screening
- The extract was subjected to preliminary phytochemical tests to find out phytoconstituents present in them. The tests were carried to detect the presence of steroids, triterpenoids, flavonoids, tannins, anthraquinones, coumarins, cardenolides, iridoids, carbohydrates and /or glycosides [15-17].
3. Statistical Analysis
- Experimental results are expressed as mean ± standard deviation (SD). All results are means of three replicates. SPSS 16 version was used for the statistical analysis and Microsoft excel program (2010).
4. Results and Discussion
4.1. Determination of Total Flavonoidal Content
- The basic principle in the Aluminum chloride colorimetric method is due to formation of acid stable complexes with the C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonols, also Aluminum chloride forms acid labile complexes with the ortho- dihydroxyl groups in the A– or B-ring of flavonoids. This complexes measured spectrophotometrically at λmax 415nm [18]. In this work; the total flavonoids of the total extract obtained from the aerial parts of were calculated from the equation of the standard plot as follow; Absorbance = 0.0094 × total flavonoid [μg QE/mg of dry extract] + 0.039 (R2 = 0.9963).
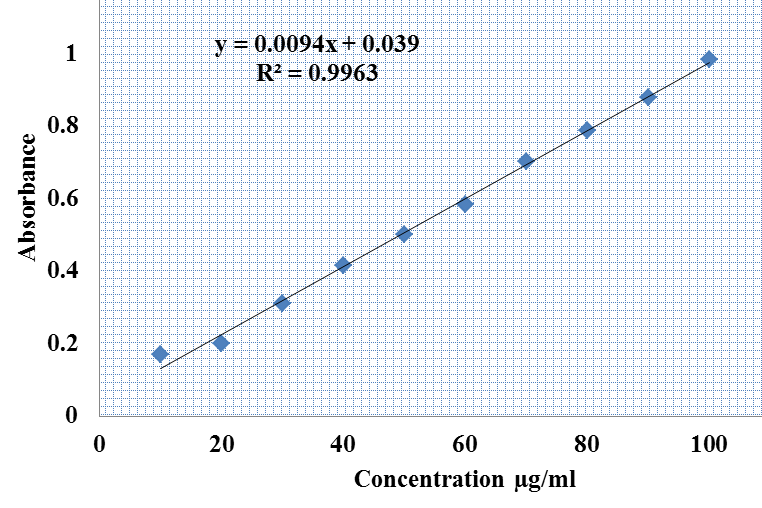 | Figure 1. Calibration curve of standard quercetin for determination of total flavonoids content in total extract of Ficus pyriformis aerial parts |
|
4.2. DPPH Radical Scavenging Activity (DPPH• assay)
- Free radicals can be defined as any molecular species capable of independent existence that contains an unpaired electron in an atomic orbital such as Reactive Oxygen Species(ROS) like (superoxide anion (O2•–), hydroxyl (•OH ), peroxy (ROO•), and alkyl radical ( RO• ) which may attack biological macromolecules, giving rise to protein, lipid, and DNA damage, cell aging, and cancer [19]. Antioxidants scavenge or quench free radical. Antioxidant activity deals with the kinetic of the reaction between antioxidant and the free radicals that scavenges or quenches [20]. In the present study; the antioxidant activity of Ficus pyriformis aerial parts total extract and its fractions were determined using DPPH• method. The DPPH• method allows a direct investigation of the ability for the extract or antioxidant to donate hydrogen and/or electrons to quench the DPPH• radical.
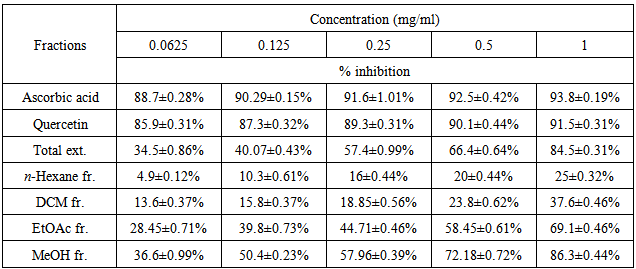
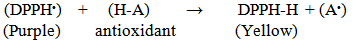 As the radical is quenched, the color of the solution changes from a deep purple to a light yellow and the absorbance at 517 nm decreases [13]. As a result (table 2, figures 2, 3, 4, 5, 6 and 7) indicated good scavenging activity of the total extract, and some fractions towards DPPH• in comparison with reference standards (ascorbic acid and quercetin). The MeOH fraction showed maximum activity in comparison with total extract and other fractions, followed by EtOAc, DCM and n-hexane fractions respectively. The obtained antioxidant activity of the total extract and fractions (Tab 2, Figures 2, 3, 4, 5, 6 and 7) are closely related to the presence of poly-phenolic compound such as flavonoids. The presence of ortho-dihydroxyl of the B-ring (3’, 4’-di OH) of the flavonoid molecule which confers high stability to the flavonoid phenoxy radical, C2-C3 double bond in conjugation with 4-oxo group of the ring C participates in radical stabilization via electron delocalization over all three ring system. The presence of both 3- and 5- hydroxyl moiety of the rings C and A, play an important role in radical scavenging activity of the flavonoids [21, 22].
As the radical is quenched, the color of the solution changes from a deep purple to a light yellow and the absorbance at 517 nm decreases [13]. As a result (table 2, figures 2, 3, 4, 5, 6 and 7) indicated good scavenging activity of the total extract, and some fractions towards DPPH• in comparison with reference standards (ascorbic acid and quercetin). The MeOH fraction showed maximum activity in comparison with total extract and other fractions, followed by EtOAc, DCM and n-hexane fractions respectively. The obtained antioxidant activity of the total extract and fractions (Tab 2, Figures 2, 3, 4, 5, 6 and 7) are closely related to the presence of poly-phenolic compound such as flavonoids. The presence of ortho-dihydroxyl of the B-ring (3’, 4’-di OH) of the flavonoid molecule which confers high stability to the flavonoid phenoxy radical, C2-C3 double bond in conjugation with 4-oxo group of the ring C participates in radical stabilization via electron delocalization over all three ring system. The presence of both 3- and 5- hydroxyl moiety of the rings C and A, play an important role in radical scavenging activity of the flavonoids [21, 22].
|
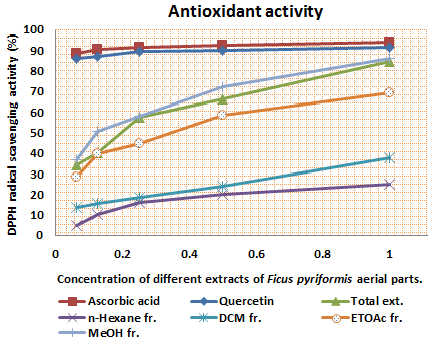 | Figure 2. Scavenging activity of extracts with different concentrations in comparison with standards |
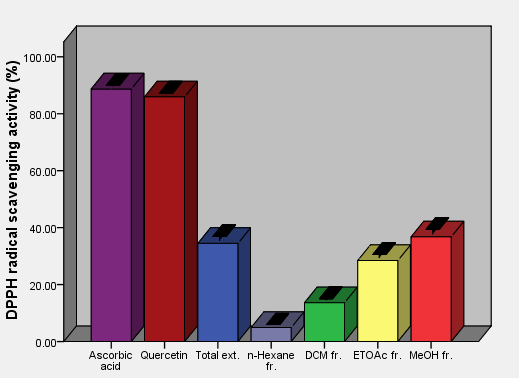 | Figure 3. DPPH free radical scavenging activity of aerial parts total extract and fractions of Ficus pyriformis in concentration (0.0625 mg/ml) in comparison with standards |
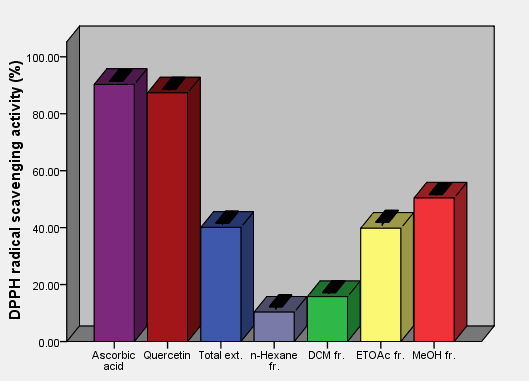 | Figure 4. DPPH free radical scavenging activity of aerial parts total extracts and fractions of Ficus pyriformis in concentration (0.125 mg/ml) in comparison with standards |
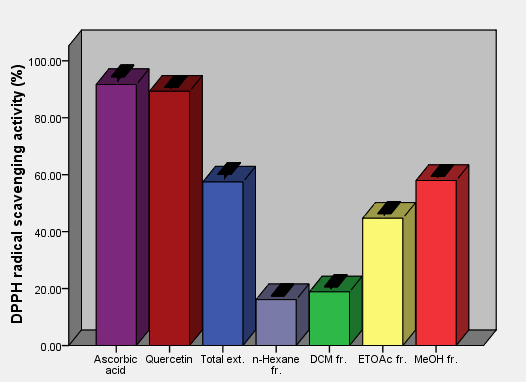 | Figure 5. DPPH free radical scavenging activity of aerial parts total extracts and fractions of Ficus pyriformis in concentration (0.25 mg/ml) in comparison with standards |
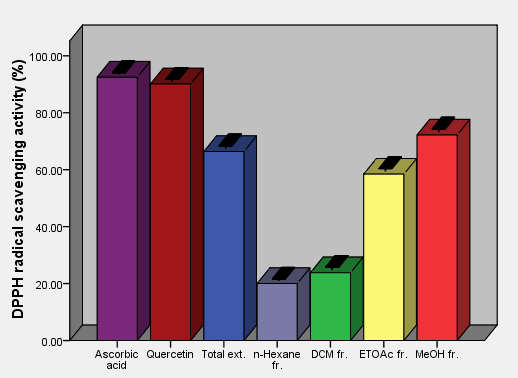 | Figure 6. DPPH free radical scavenging activity of aerial parts total extracts and fractions of Ficus pyriformis in concentration (0.5 mg/ml) in comparison with standards |
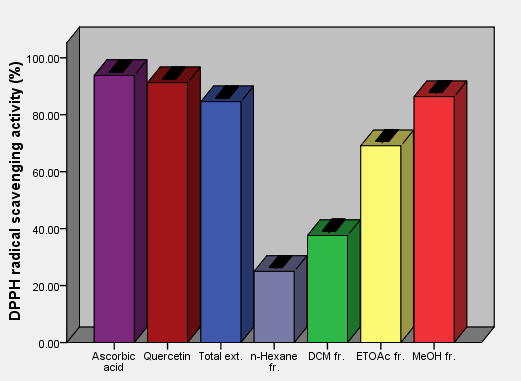 | Figure 7. DPPH free radical scavenging activity of aerial parts total extracts and fractions of Ficus pyriformis in concentration (1 mg/ml) in comparison with standards |
4.3. Phytochemical Screening
- Preliminary phytochemical screening of the total extract showed the presence of steroids, triterpenoids, flavonoids, tannins, carbohydrates and or glycosides. Flavonoids and tannins contributed to have an antioxidant activity [8, 9].Value are expressed as mean ± SD; n = 3, Total ext. = total extract, n-Hexane Fr. = Hexane fraction, DCM Fr. = Dichloromethane fraction, EtOAc fr. = Ethyl acetate fraction, MeOH fr. = Methanol fraction.
5. Conclusions
- For the first time the evaluation of the antioxidant activity of the aerial parts of Ficus pyriformis was done along with its total flavonoids content. The MeOH fraction and total extract of Ficus pyriformis aerial parts showed highest antioxidant activity followed by EtOAc, DCM and n-hexane fraction respectively in comparison with reference standards and this attributed to polyphenolic compound as flavonoids and tannins which known to possess an antioxidant activity [14, 22, 23].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML