-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(5): 155-165
doi:10.5923/j.chemistry.20140405.03
Novel Synthesized Surfactants Based on Palm Oil and Monoethanolamine as Corrosion Inhibitors for Mild Steel in CO2 Environments
I. T. Ismayilov1, 2, Hany M. Abd El-Lateef3, V. M. Abbasov1, E. N. Efremenko2, L. I. Aliyeva1, S. F. Akhmadbeyova1
1Mamedaliev Institute of Petrochemical Processes, National Academy of Sciences of Azerbaijan, Baku, Azerbaijan
2Faculty of Chemistry, Lomonosov Moscow State University, Moscow, Russia
3Chemistry Department, Faculty of Science, Sohag University, Sohag, Egypt
Correspondence to: Hany M. Abd El-Lateef, Chemistry Department, Faculty of Science, Sohag University, Sohag, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Potentiodynamic polarization and linear polarization resistance corrosion rate measurements were carried out to study the corrosion inhibition of mild steel in 1% NaCl saturated with CO2 by some novel surfactants type of fatty acids derivatives synthesized based on palm oil and monoethanolamine. The surface tension at 298 K was measured; the critical micelle concentration (CMC) and some surface active parameters were calculated. The inhibition efficiency was found to increase with increasing concentration. The results obtained show that the prepared surfactants act as mixed-type inhibitors. The inhibitive effect of these compounds was explained on the basis of adsorption on the metal surface. The adsorption process follows Langmuir adsorption isotherm. Some activated thermodynamic parameters were computed and discussed.
Keywords: Surfactants, Mild steel, Palm oil, Corrosion inhibition, CO2-saturated brine
Cite this paper: I. T. Ismayilov, Hany M. Abd El-Lateef, V. M. Abbasov, E. N. Efremenko, L. I. Aliyeva, S. F. Akhmadbeyova, Novel Synthesized Surfactants Based on Palm Oil and Monoethanolamine as Corrosion Inhibitors for Mild Steel in CO2 Environments, American Journal of Chemistry, Vol. 4 No. 5, 2014, pp. 155-165. doi: 10.5923/j.chemistry.20140405.03.
Article Outline
1. Introduction
- Mild steel is widely applied as the constructional materials in many industries due to its excellent mechanical properties and low cost. Carbon dioxide (CO2) corrosion is one the most studied of corrosion in oil and gas industry. This is generally due to the fact that the crude oil and natural gas from the oil reservoir / gas well usually contains some level of CO2. The major concern with CO2 corrosion in oil and gas industry is that CO2 corrosion can cause failure on the equipment especially the main down hole tubing and transmission pipelines and thus can disrupt the oil/gas production [1-3]. Because of the general aggression of solutions saturated with CO2, inhibitors are commonly used to reduce the corrosive attack on metallic materials [4]. The selection of inhibitor is controlled by its economic availability, its efficiency to inhibit the substrate material and its environmental side effects. So that, most of the excellent inhibitors for corrosion of steel in carbon dioxide environments are organic compound containing nitrogen, oxygen and/or sulphur atoms [5-15]. The inhibiting action of these compounds is attributed as a first stage, to the adsorption of the additives to the metal/solution interface. The adsorption process depends upon the nature and surface charge of the metal, the type of aggressive media, the structure of the inhibitor and the nature of its interaction with the metal surface.Surfactants can be easily synthesized from relatively cheap raw materials, nontoxic and have surface active property. Surfactants based on fatty acids were used as corrosion inhibitors for steel in CO2-saturted solutions [16]. They inhibit the corrosion by the adsorption on the steel surface. The aim of this investigation is to examine the inhibitory effect of some surfactants based on palm oil toward the corrosion of mild steel corrosion in CO2-saturated 1% NaCl solution. Linear polarization resistance corrosion rate and potentiodynamic polarization techniques were used in this work to evaluate the inhibition efficiency of the tested compounds.
2. Experimental methods
2.1. Synthesis of Surfactants
- Palm oil was reacted with monoethanolamine for 14 hours at 423-433 K. These processes produce fatty acid monoethanolamine amide. Based on the last prepared compound sulfating syntheses were performed. The Sulfating process was done in 500 ml ground glass three-neck flask equipped with a mechanical stirrer, a thermometer with a temperature controller, were charged with 100 gm fatty acid and then H2SO4 was added drop by drop at the reaction temperature in the range from 343 to 348 K with stirring for 7 h. The product is sulfated fatty acid monoethanolamine amide. Five types from surfactants were synthesized in high purity by the following composition: [R-CH-(OSO3M)-CONH-CH2-CH2-OH] (where M = Na, K, NH4, –NH-CH2-CH2-OH and –N-(CH2-CH2-OH)2). List of the synthesized surfactants are shown in Table 1. The chemical structure of the synthesized surfactants was characterized by using FT-IR, Spectrum BX spectrometer using KBr disks.
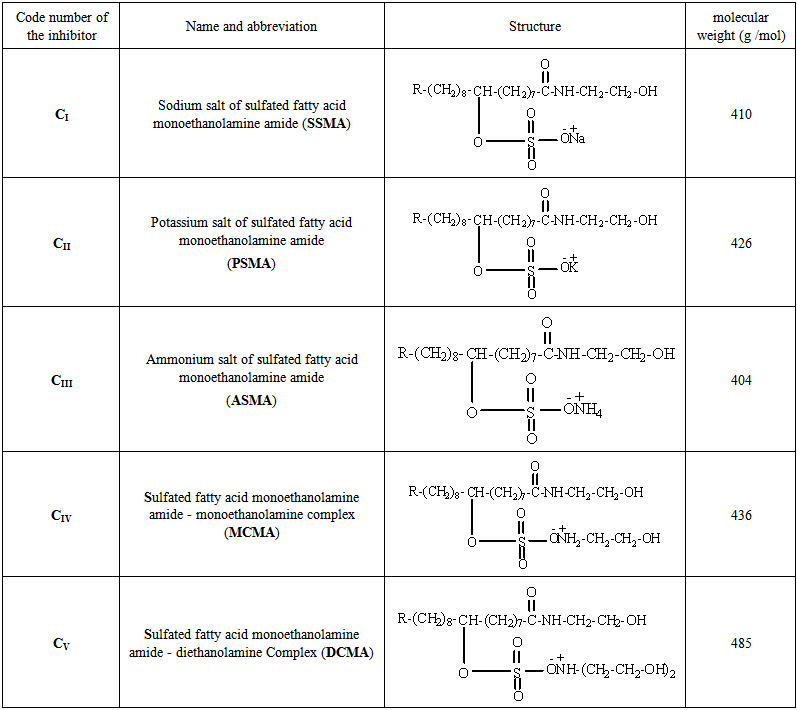 | Table 1. List of the prepared surfactants includes, code number, name and structure |
2.2. Chemical Composition of Mild Steel Alloy
- Mild steel was used for this study has the following composition: C 0.18%, Si 0.17%, Mn 0.70%, P 0.011%, S 0.03%, Ni 0.01%, Cr 0.01% and Fe balance.
2.3. Corrosion Measurements
- The aggressive solution, 1% NaCl, was prepared by dissolving of analytical grade NaCl in distilled water. The concentration range of the prepared surfactants was from 25 to 100 ppm used for corrosion measurements. All inhibitors solutions were prepared using a mixture from distilled water and alcohol in a different ratio.The measurements were performed on the rotating cylinder electrode as a working electrode. This working electrode was used for one time without polishing. The reference electrode was Ag/AgCl Electrode to which all potentials are referred.The extrapolation of cathodic and anodic Tafel lines was carried out in a potential range ±100 mV with respect to corrosion potential (Ecorr) at scan rate of 1 mV/s. To remove any surface contamination and air formed oxide, the working electrode was kept at−1500 mV (Ag/AgCl) for 5 min in the tested solution, disconnected shaken free of adsorbed hydrogen bubbles and then cathodic and anodic polarization was recorded. ACM Gill AC instrument connected with a personal computer was used for the measurements. LPR test has been performed in brine saturated with CO2 at 323 K, in turbulence fluid stream during 20 hours. The prepared 1% NaCl was stirred by a magnetic stirrer for 60 min in 4000 ml glass beaker. The prepared solution poured into the 4 glass beakers (1000 ml for each one). Then these beakers were placed on a heater at 50℃ for 1 hour under a pressure of 0.9 bars. The solution was saturated with carbon dioxide (pH=5.7). After that, the electrodes were placed in the medium and are connected through a potentiometer ACM GILL AC. The surface of working electrode is cleaned by acetone before using, these electrodes are using for one time. After 1 hour, except for 1 beaker, the remaining 3 is fed with the suitable amount of inhibitor and continued supply of CO2 under pressure of 0.9 bar until the end of the experiment. The potential of the working electrode was varied by a CoreRunning programme (Version 5.1.3.) through an ACM instrument Gill AC. The CoreRunning programme converts a corrosion current in mA/cm2 to a corrosion rate in mm/year. A cylindrical mild steel rod of the composition 080A15 GRADE STEEL was used as a working electrode. Gill AC technology allows measure DC and AC signals using standard Sequencer software. A small sweep from typically –10 mV to +10 mV at 10 mV/min around the rest potential is performed.
2.4. Surface Tension Measurements
- The surface tension (γ) of the investigated surfactants was measured using (DuNouy Tensiometer, Kruss Type 8451) for various concentrations of the prepared surfactants.
3. Results and Discussion
3.1. Linear Polarization Corrosion Rate (LPR Bubble Test)
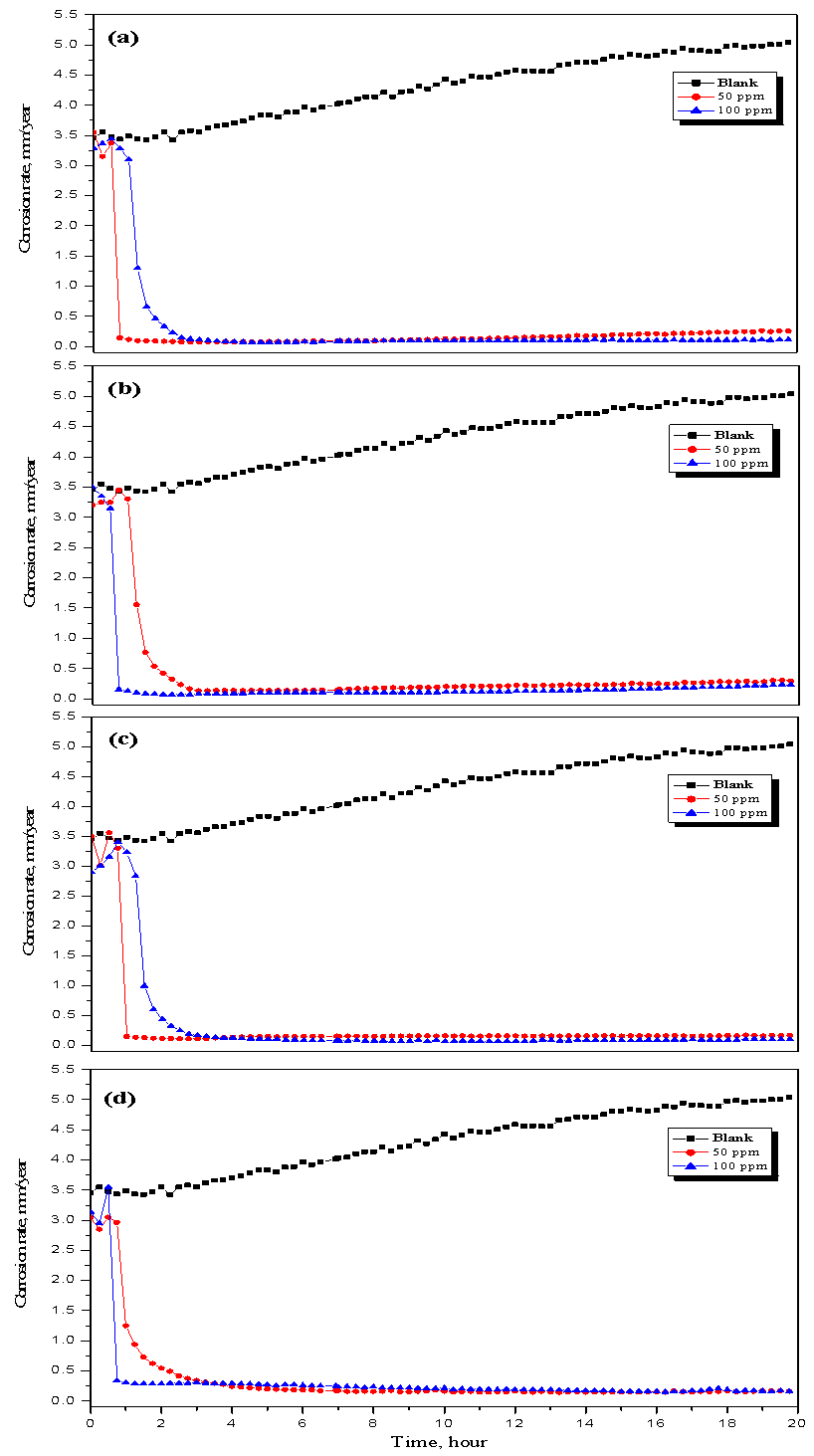
- The linear polarization resistance corrosion rate test involved evaluating the corrosion of mild steel in CO2-saturated 1 % NaCl solution at 323 K. Fig. 1 a, b, c and d show that, the change in corrosion rate (CR) with time for mild steel in CO2-saturated 1 % NaCl solution containing different concentrations for surfactants (a) CI, (b) CII, (c) CIII and (d) CV at 323 K. The inhibitor was added after 1 hour of exposure because at this time the corrosion potential got stable, allowing the measurement of the CR prior the addition of the inhibitor. The initial corrosion rate, without inhibitor was measured to be between 4.18 and 4.9 mm y-1. From Fig. 1 it can be observed that, in the baseline test (no inhibitor addition), CO2 corrosion rate steadily increase with time since the corrosion process leaves iron carbide (Fe3C) left on the mild steel surface, which increases surface area of cathodic reaction and further increases the corrosion rate [17-19]. The increase in the corrosion rate had been attributed to the decrease in pH value from 6.77 to 5.70 (more acidic) after saturation with CO2. The inhibition efficiency (η%) and surface coverage (θ) were calculated according to the following equations [3]:
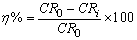 | (1) |
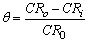 | (2) |
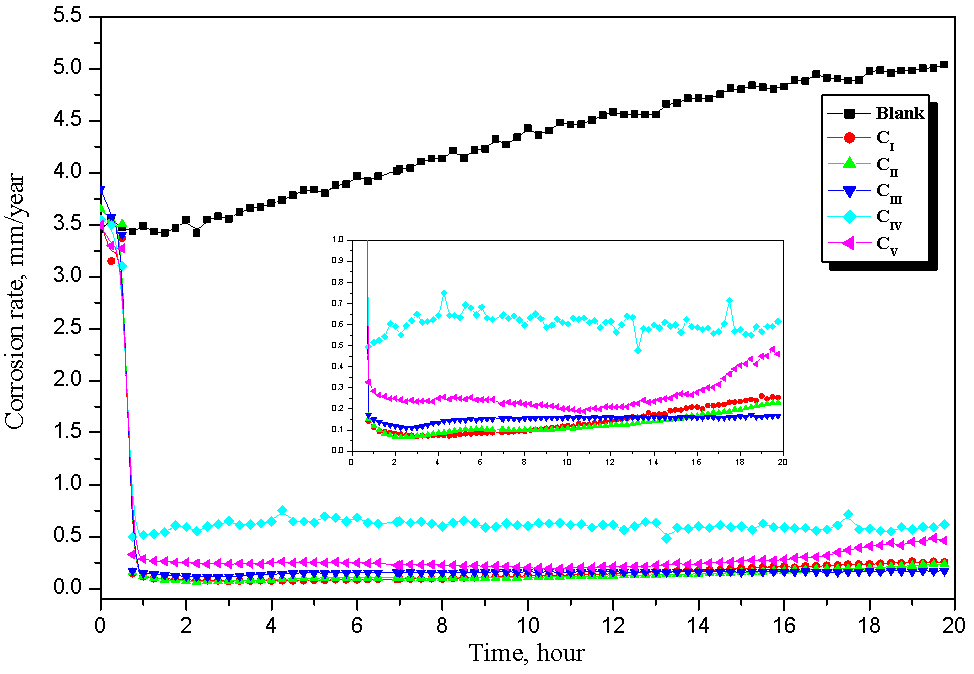 | Figure 2. Variation of the Corrosion rate with time for carbon steel in CO2-saturated 1% NaCl solution containing 100 ppm of different inhibitors at 323 K |
3.2. Potentiodynamic Polarization Measurements
- The effect of addition of the prepared surfactants of fatty acid derivatives on the anodic and cathodic polarization curves for mild steel in CO2-saturated brine solution at 323K was studied. The effect of increased concentration of compound CI is shown in Fig. 3 as an example of the studied surfactants. Similar results were obtained for the other inhibitors (not shown). The dependence of the degree of surface coverage (θ) and the inhibition efficiency (η%) on the concentration of the inhibitor were calculated using [16]:
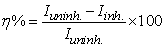 | (3) |
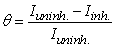 | (4) |
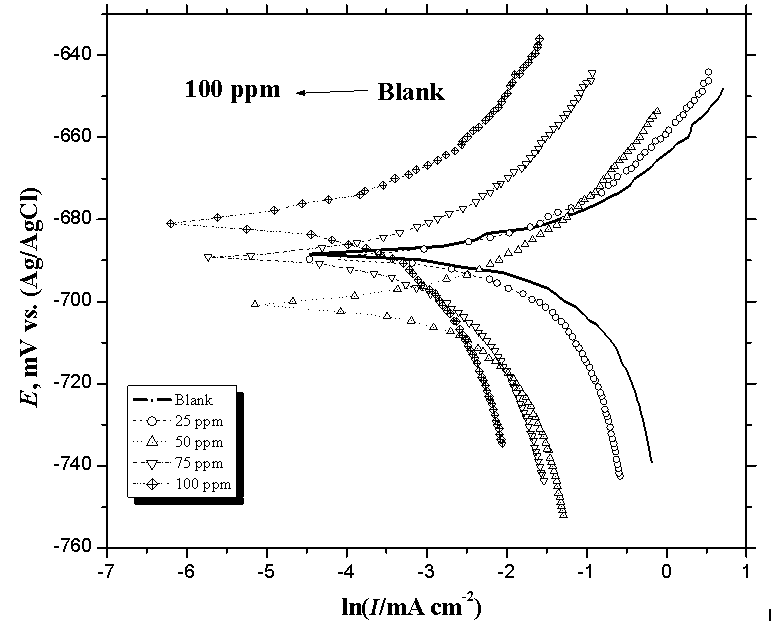 | Figure 3. Potentiodynamic polarization plots of carbon steel electrode obtained in CO2-saturated 1% NaCl solution containing different concentration of inhibitor (CI) at 323 K |
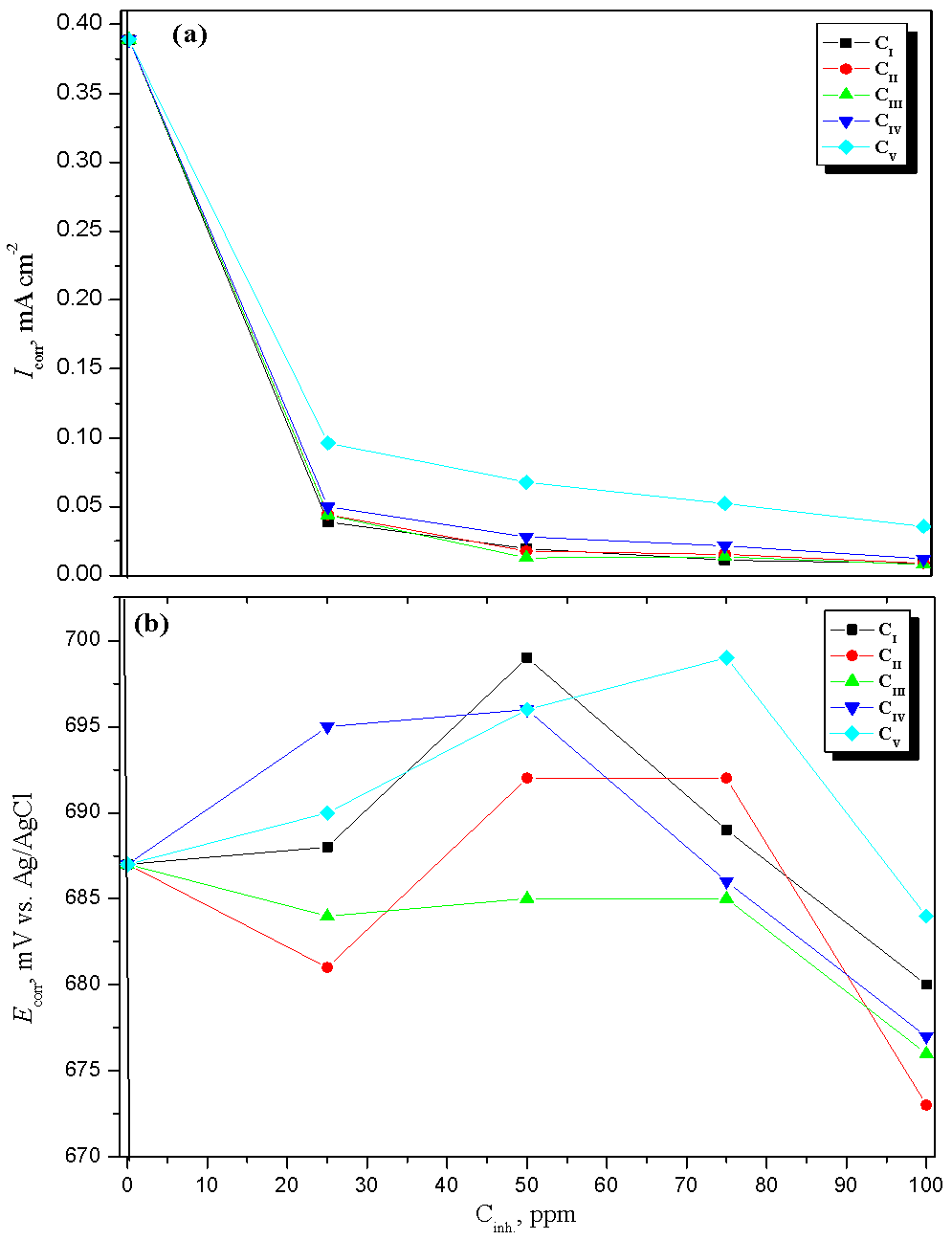 | Figure 4. Comparison between (a) corrosion current density, Icorr and (b) corrosion potential, Ecorr, of mild steel alloy in CO2-saturated solution containing different concentrations of the prepared surfactants at 323 |
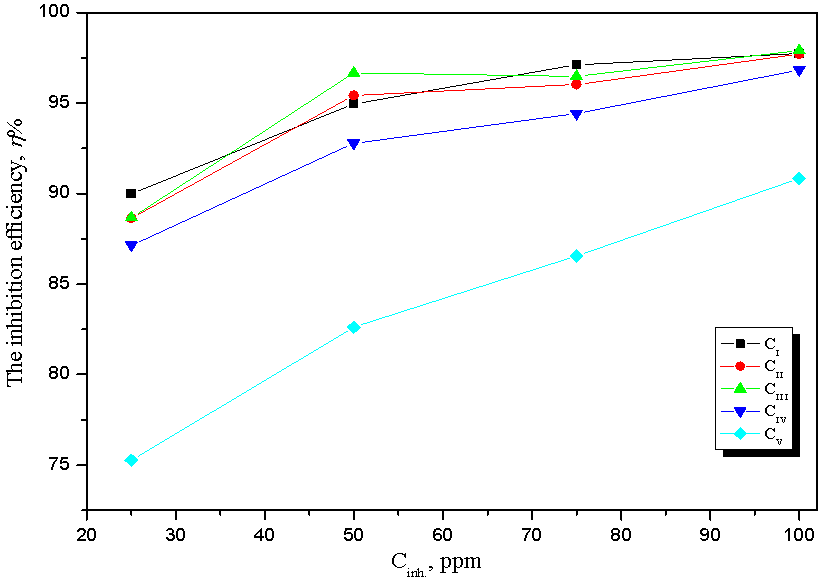 | Figure 5. Comparison between inhibition efficiency of mild steel alloy in CO2-saturated solution containing different concentrations of the prepared surfactants at 323 K |
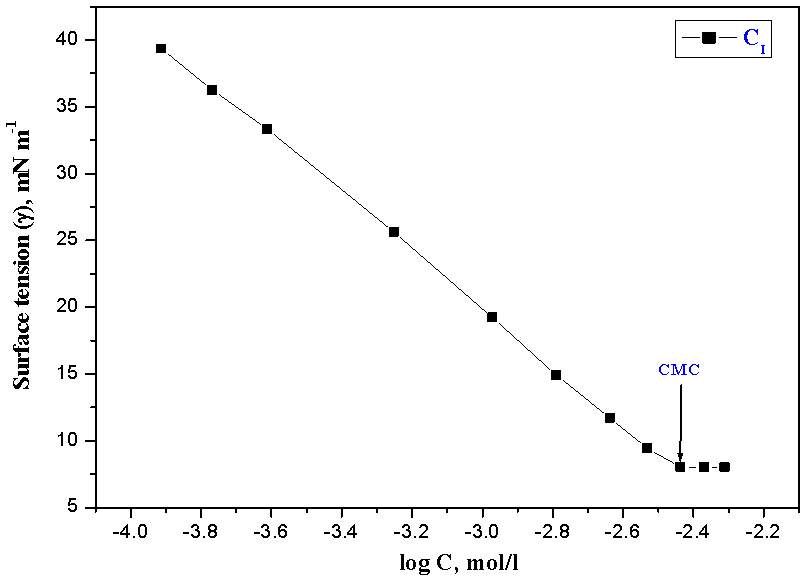 | Figure 6. Variation of surface tension vs. log C of the inhibitor CI at 298 K |
3.3. Adsorption Isotherms and Thermodynamic Parameters for the Corrosion Process
- The values of the degree surface coverage θ were evaluated at different concentrations of the prepared surfactant compounds in CO2-saturated solution at 323 K. The values of θ have been used to explain the best isotherm to determine the adsorption process. For obtaining the best description of adsorption behavior of the inhibitor, attempts were made to fit θ values to various isotherms including Frumkin, Temkin, Freundlich and Langmuir adsorption isotherms. By far the best fit was obtained with the Langmuir isotherm and can be represented using the following equation [1]:
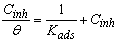 | (5) |
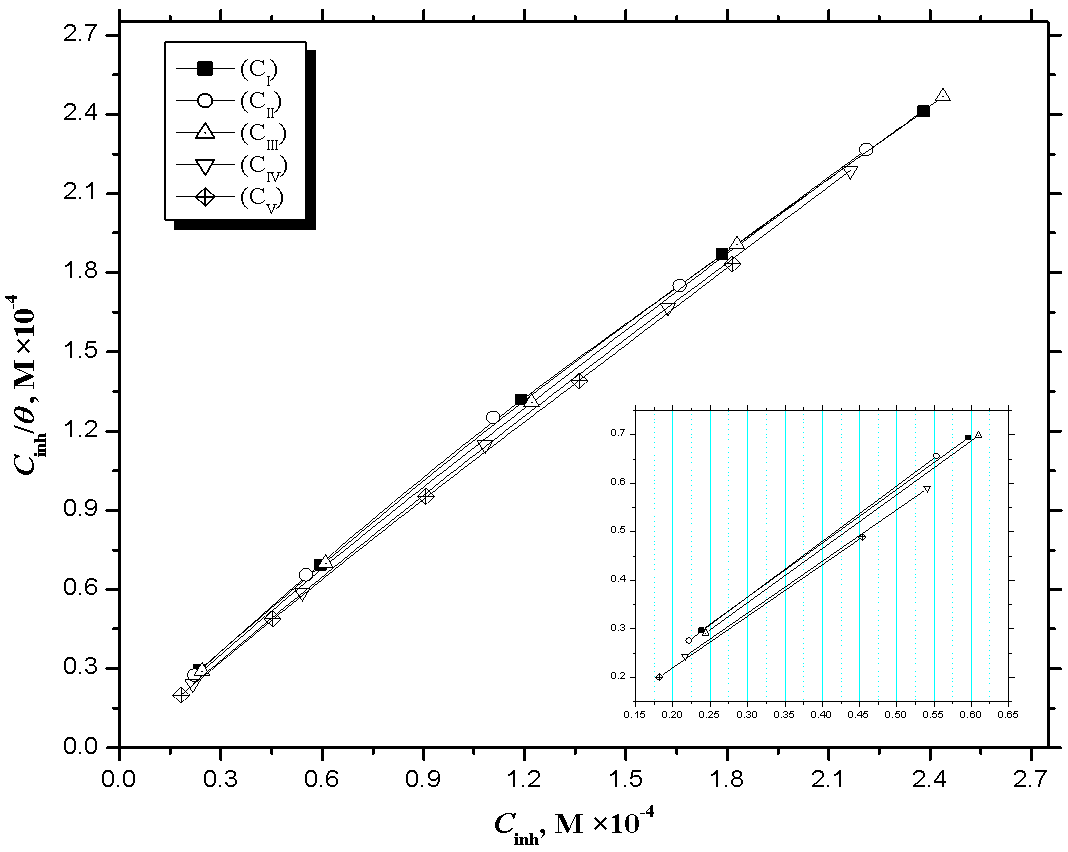 | Figure 7. Langmuir adsorption isotherm (Ci/θ vs. Ci) fitting of the obtained potentiodynamic polarization data for mild steel in CO2 saturated brine containing various concentrations of inhibitors at 323 K |
|
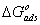 ) calculated from [16]:
) calculated from [16]: 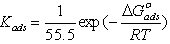 | (6) |
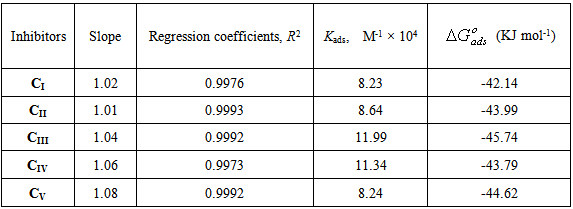
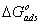 provide information about the mechanism of the inhibitor molecules adsorption at the metal surface. The negative values of
provide information about the mechanism of the inhibitor molecules adsorption at the metal surface. The negative values of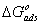 , are consistent with the spontaneity of the adsorption process and the stability of the adsorbed layer on the mild steel surface. Generally, values of
, are consistent with the spontaneity of the adsorption process and the stability of the adsorbed layer on the mild steel surface. Generally, values of 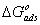 up to -20 kJ mol-1 are consistent with physisorption, while those around -40 kJ mol-1 or higher are associated with chemisorption as a result of the sharing or transfer of electrons from organic molecules to the metal surface to form a coordinate bond [1-3, 16]. In the present study, the values of
up to -20 kJ mol-1 are consistent with physisorption, while those around -40 kJ mol-1 or higher are associated with chemisorption as a result of the sharing or transfer of electrons from organic molecules to the metal surface to form a coordinate bond [1-3, 16]. In the present study, the values of 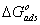 obtained for studied surfactants on mild steel in CO2-saturated solution more than -40 kJ mol-1 (Table 3). These results indicate that the adsorption mechanism of surfactants on mild steel in CO2 saturated solution is typical chemisorptions at the studied temperature.
obtained for studied surfactants on mild steel in CO2-saturated solution more than -40 kJ mol-1 (Table 3). These results indicate that the adsorption mechanism of surfactants on mild steel in CO2 saturated solution is typical chemisorptions at the studied temperature.4. Conclusions
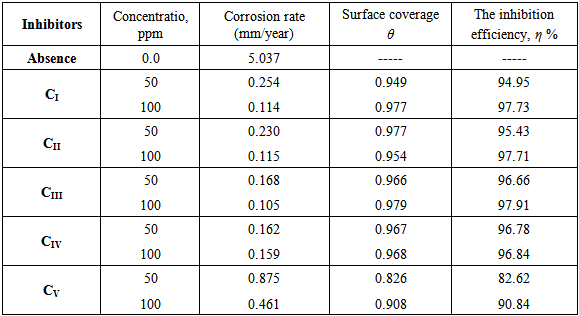
- 1. The synthesized surfactants of fatty acids derivatives are considered as good inhibitors for mild steel corrosion in CO2-saturated brine.2. The inhibition efficiency was found to increase by increasing the inhibitor concentrations. The inhibition efficiencies are 97.73%, to 97.71%, 97.91%, 96.84% and 90.84% for inhibitors CI, CII, CIII, CIV and CV respectively at 100 ppm.3. The Ecorr values of all synthesized surfactants were shifted slightly toward both cathodic and anodic directions and did not show any definite trend in CO2-saturated brine. This may be contributed to the mixed-type behavior of the studied inhibitors.4. The inhibitive action of surfactants compounds is due to the adsorption on the steel surface.5. The adsorption of cationic surfactants compounds on the steel surface follows Langmuir adsorption isotherm.
References
| [1] | Abbasov V. M., Abd El-Lateef H. M., Aliyeva L. I., Ismayilov I. T. , Qasimov E. E. Efficient Complex Surfactants from the Type of Fatty Acids as Corrosion Inhibitors for Mild Steel C1018 in CO2-Environments. Journal of the Korean Chemical Society, 2013, 57, 25-34. |
| [2] | Abbasov V. M., Abd El-Lateef H. M., Aliyeva L.I., Ismayilov I.T. Application of Some Surfactants Based On Corn Oil as Corrosion Inhibitors for Carbon Steel in CO2 Environments, NACE corrosion 2013, Florida, USA, paper No. 2129: p.1-10. |
| [3] | Ismayilov I.T., Abd El-Lateef, H. M., Abbasov V.M., Aliyeva L.I., Efremenko E.N., Mamedxanova S.A. Adsorption and corrosion inhibitive properties of novel surfactants in the series of fatty acids based on palm oil on carbon steel in CO2-containing solution, International Research Journal of Pure & Applied Chemistry, 2014, 4, 299-314. |
| [4] | Rozenfeld I.L., Corrosion Inhibitors; McGraw-Hill. New York, 1981, p.182. |
| [5] | Abdallah M., Asghar B. H., Zaafarany I, Fouda A.S. The Inhibition of Carbon Steel Corrosion in Hydrochloric Acid Solution using Some Phenolic Compounds, Int.J. Electrochem Sci., 2012, 7(1), 282-304. |
| [6] | Abdel-Aal M., Morad M.S. Inhibiting effects of some quinolines and organic phosphonium compounds on corrosion of mild steel in 3M HCl solution and their adsorption characteristics, Br. Corros. J., 2001, 36, 250. |
| [7] | Abdallah M., El-Naggar M.M. Cu+2 cation+ 3,5-dimethyl pyrazole mixture as a corrosion inhibitor for carbon steel in sulfuric acid solution, Materials Chem. and Phys. 2001, 71, 291. |
| [8] | Bonklah M., Quassini A., Hammouti B., El-Idrissi A.. Corrosion inhibition of steel in sulphuric acid by pyrrolidine derivatives, Appl. Surf. Sci., 2006, 252, 2178. |
| [9] | Abdallah M., Megahed H.E, Motae M.S. Inhibiting effect of Ni2+ cation+ 3- methyl pyrazolone as a corrosion inhibitor for carbon steel in sulfuric acid solution, Mater. Chem. and Phys, 2009,118, 111-117. |
| [10] | Abd El-Maksoud S.A., Fouda A.S. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium, Mater. Chem. and Phys. 2005, 93 84-90. |
| [11] | Abd El-Rehim S.S., Ibrahim M.A.M., Khaled K.F. 4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution, J. Appl. Electrochem. 1999, 29, 593-599. |
| [12] | Abdallah M., Moustafa M. E. Inhibition of Acidic Corrosion of Carbon Steel by Some Mono and Bis Azo Dyes Based on 1,5-Dihydroxynaphthalene, Annali Di Chimica (Italy), 2004, 94 601. |
| [13] | Popora A., Sokolova E., Raicheva S., Christov M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives, Corros. Sci. 2003, 45, 33-58. |
| [14] | Sobhi M., Abdallah M., Khairou K. Sildenafil citrate (Viagra) as a corrosion inhibitor for carbon steel in hydrochloric acid solutions Monatshefte fur Chemie, 2012, 143, 1379-1387. |
| [15] | Abdallah M., Zaafarany I., Khairou K. S., Sobhi M. Inhibition of Carbon Steel Corrosion by Iron (III) and Imidazole in Sulfuric Acid, Int. J. Electrochem Soc., 2012, 7(2), 1564. |
| [16] | Abd El-Lateef H.M., Abbasov V.M., Aliyeva L.I., Qasimov E.E., Ismayilov I.T., Inhibition of carbon steel corrosion in CO2-saturated brine using some newly surfactants based on palm oil: Experimental and theoretical investigations, Materials Chemistry and Physics 2013, 142, 502-512. |
| [17] | Staicopolus N. The Role of Cementite in the Acidic Corrosion of Steel, J. Electrochem. Soc. 1963, 110, 1121-1124. |
| [18] | Crolet J., Thevenot N., Nesic S. Role of Conductive Corrosion Products in the Protectiveness of Corrosion Layers, Corrosion, 1998, 54, 194-203. |
| [19] | Videm K., Kvarekvaal J., Perez T., Fitzsimons G. Corrosion of Carbon Steel in Carbon Dioxide-Saturated Solutions Containing Small Amounts of Hydrogen Sulfide, Corrosion, 1995, 51,260-269. |
| [20] | López D. A., Simison S. N., de Sánchez S. R. Inhibitors performance in CO2 corrosion: EIS studies on the interaction between their molecular structure and steel microstructure, Corros. Sci. 2005, 47, 735-755. |
| [21] | Akbarzadeh E., Ibrahim M. N. M., Rahim A. A. Corrosion Inhibition of Mild Steel in Near Neutral Solution by Kraft and Soda Lignins Extracted from Oil Palm Empty Fruit Bunch, Int. J. Electrochem. Sci., 2011, 6, 5396 – 5416. |
| [22] | El-Sayed A., Mohran H. S., Abd El-Lateef H.M., The inhibition effect of 2,4,6-tris (2-pyridyl)-1,3,5-triazine on corrosion of tin, indium and tin–indium alloys in hydrochloric acid solution Corros. Sci. 2010, 52, 1976–1984. |
| [23] | Shalaby M. N., Osman M.M., El-Feky A.A. Effect of some organic surfactants on corrosion inhibition of steel in sea water, Anti-corros. Meth. Mat. 1999, 46, 254-260. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML