Violeta Jevtovic1, Jasmina M. Jovanovic-Mirkovic2
1Department of Biological Sciences and Chemistry, College of Art & Sciences, University of Nizwa, Oman
2College of Health Studies, Cuprija, Serbia
Correspondence to: Violeta Jevtovic, Department of Biological Sciences and Chemistry, College of Art & Sciences, University of Nizwa, Oman.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This work describes synthesis, physico-chemical properties and biological activity of a series of five novel cobalt (II) and (III) complexes with pyridoxal-S-methylisotiosemicarbazone. This series of Co complexes is represented by geometry, estimated according to the magnetic characteristics of the complexes, color, IR and microanalysis. Furthermore, their expressed biological activity in terms of strong inhibition of cell proliferation towards MCF7 and MDA-MB-231breast adenocarcinoma cell lines is discussed.
Keywords:
Series of Co(II) and (III) complexes, Pyridoxal-S-methylisotiosemicarbazone ligand, Synthesis, physico-chemical properties, Biological activity
Cite this paper: Violeta Jevtovic, Jasmina M. Jovanovic-Mirkovic, A Series of Co(II) and Co(III) Complexes Incorporating Pyridoxal-S-methylisotiosemicarbazone: Synthesis, Physico-chemical Characteristic and Biological Activity, American Journal of Chemistry, Vol. 4 No. 5, 2014, pp. 131-136. doi: 10.5923/j.chemistry.20140405.01.
1. Introduction
Isothiosemicarbazones (ITSC) can act as biologically active antibacterial agents [1] and are excellent chelating ligands of different denticity resulting in the synthesis of a large number of transition metal complexes containing these ligands [2, 3]. Several of these complexes, due to their stability and intense color, have been suggested as analytical reagents [4]. Furthermore, complexes incorporating ITSC-based ligands exhibit a wide variety of biologically important properties, such as antiviral, antitumor and anti-inflammatory activities [5, 6]. Dehydration of ITSC with pyridoxal moiety (3-hydroxy-5-hydroxymethyl-2-methyl-pyridine-4-carbaldehyde) results in the formation of Schiff base ligands PLSC and PLITSC, respectively [7]. The synthesis, physical properties, structural analysis, as well as the biological activities of several transition metal complexes incorporating PLITCS [8-11] have already been described. The tridentate coordination mode is predominant with regards to PLITSC which can adopt three different forms in the coordination sphere of a transition metal, namely neutral (but zwitterionic), monoanionic (hydrazine deprotonation) and dianionic (both pyridinium and hydrazine deprotonation) forms (see Sheme 1).The complexes incorporating the neutral form of PLITSC (L’), such as [Fe(ITSCPL)Cl3].H2O [9], are most predominant, while several examples in which the ligand is found in monoanionic (e.g.[Fe(L’-H)2]OAc.2H2O[9]) and dianionic (e.g. [Cu(L’-2H)NH3]. H2O.MeOH [7]) forms are also reported.Thus, we wish to report the new complexes ITSCPL with cobalt: [Co(H2L)Cl2].2H2O(1), [Co(H2L)(HL)]Cl2.4H2O(2), [Co(HL)2]Cl.2H2O(3), [Co(HL)(L)]CH3.OH(4) and [Co(L)(NH3)3]NO3.3H2O(5). Also, physical and chemical properties, molar conductivity, magnetic measuring, elementary analysis and composition of complexes of these complexes are reported.
2. Experimental
2.1. Reactants
All commercially obtained reagent-grade chemicals were used without further purification, except for the ligand, which were prepared according to the previously described procedure [12].
2.2. Measurements
Elemental (C,H, and N) analysis of air-dried samples was carried out by standard micro methods in the Center for Instrumental Analysis, Faculty of Chemistry, Belgrade. Molar conductivities of the freshly prepared 1x10-3 M solution were measured on a Jenway 4010 conductivity meter. IR spectra (KBr disk) were recorded on a Thermo Nicolet (NEXUS 670 FT-IR) instrument. Magnetic susceptibility properties were measured using a magnetic susceptibility balance, Johnson Matthey Chemical Ltd.
2.3. Synthesis of Ligand and Complexes
2.3.1. Synthesis of Ligand
Pyridoxal S-methylisothiosemicarbazone (ITSCPL), is synthesized according to the reported preparation [7] involving the reaction between pyridoxal-hydrochloride (PL.HCl) and S-methylisothiosemicarbazidehydroiodide (SMeTSC.HI) in the presence of Na2CO3.10H2O and in warm water (Scheme 2).The synthesized ligand is bright-yellow, fibrous substance, partially soluble in water but with great solubility in most common organic solvents.
2.3.2. Synthesis of complex [Co(H2L)Cl2].2H2O(1)
A mixture of 0.27g (1mmol) ITSCPL and 0.28g (1.1mmol) CoCl2.6H2O was poured over with 10 cm3 EtOH and refluxed or 30 min. After cooling down bright-grimly micro-crystal product it is filtered and washing with EtOH. Yield: 0.20g. Micro-Analysis: calc. (%) for C9H16N4O5Cl2CoS:C 28.39; H 4.62; N 13,13;S 7.25. Found: C 28.59; H 4.03; N 12.74; S 7.03. 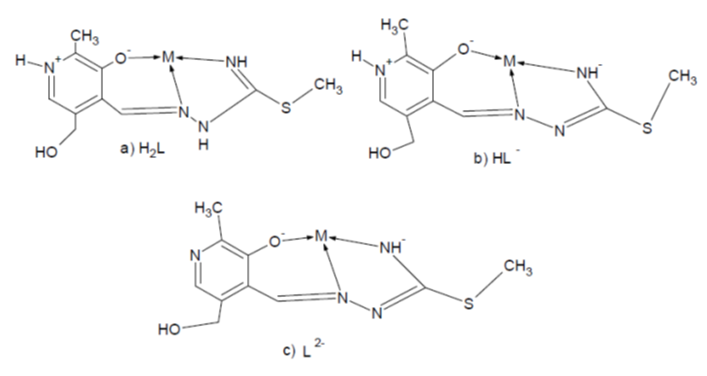 | Scheme 1. Coordination models and ligand forms: a) neutral, b) mono-and c) dianionic |
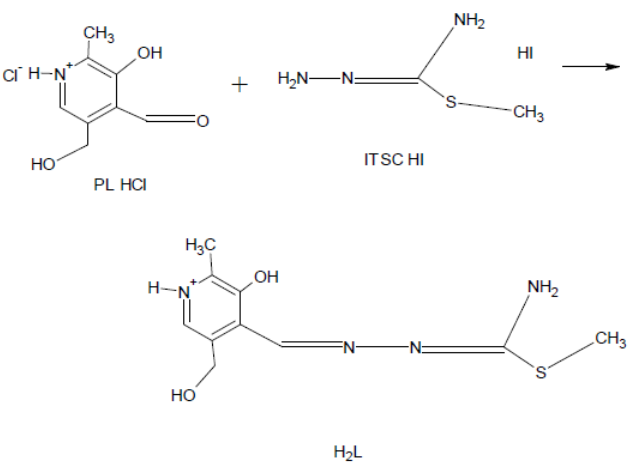 | Scheme 2. Synthesis of Pyridoxal-S-methylisothiosemicarbazone (PLITSC) |
2.3.3. Synthesis of complex [Co(H2L)(HL)]Cl2.4H2O(2)
A mixture of 0.27g (1mmol) ITSCPL and 0.28g (1mmol) CoCl2.6H2O, was poured over with 10cm3 MeOH and warmed up to dissolve all reactants. Warm mixture was filtered left for 30hours at room temperature. Separated dark-grimly crystals were filtered and washed with MeOH. Yield: 0.14g. Micro-Analysis: Calc. (%) for C18H31N8O10Cl2CoS2: C 34.67; H 5.08; N 15.83; S8.75. Found: C 34.29; H 4.85; N 15.97; S 8.63.
2.3.4. Synthesis of complex [Co(HL)2]Cl.2H2O(3)
A mixture 0.27g (1mmol) ITSCPL and 0.14g (0.5mmol) CoCl2.6H2O was poured over with 10cm3MeOH and warmed up. After cooling down bright-grimly micro-crystal product was filtered and washed with EtOH. Yield: 0.20g. Micro-Analysis: Calc. (%) for C20H30N8O6ClCoS2:C 34.75; H 4.75; N 17.59; S 10.06. Found: C 34.78; H 4.78; N 16.25; S 8.98.
2.3.5. Synthesis of complex [Co(HL)(L)]CH3.OH(4)
A mixture 0.27g (1mmol) ITSCPL and 0.28g (1mmol) Co(CH3COO)2.4H2O was poured over with 10cm3MeOH and warmed up to all dissolve the reactants. Warm mixture was filtered and left for 10 hours at room temperature. Separated dark-grimly monocrystals were filtered and washed with MeOH. Yield: 0.16g. Micro-Analysis: Calc. (%) for C21H29N8O5ClCoS2: C 42.53; H 4.76; N 19.03; S 10.45. Found: C 41.92; H 5.14; N 18.05; S 9.20.
2.3.6. Synthesis of complex [Co(L)(NH3)3]NO3.3H2O(5)
A mixture of 0.27g (1mmol) ITSCPL and 0.29g (1.mmol) Co(NO3)2 was dissolved using 7 ml of MeOH followed by immediate addition of 3ml of NH3. The mixture was filtered and the product left to crystallize. Separated dark-grimly monocrystals were filtered and washed with MeOH. Yield: 0.20g. Micro-Analysis: Calc. (%) for C10H21N8O5Cl2CoS:C 25.06; H 5.82; N 23.44; S6.95. Found: C 24.07; H 5.58; N 24.54; S 6.26.
2.4. Biological Activity
The compound was evaluated for its in vitro cytotoxicity towards estrogen receptor positive and estrogen receptor negative breast adenocarcinoma cell lines (MCF7 and MDA-MB-231, respectively). Cytototoxic activity was evaluated by colorimetric sulforhodamine B (SRB) assay, after exposure of cells to tested compound for 24 hand 72 h.The cells were grown in Dulbecco’s Modified Eagle’s Medium with 4.5% of glucose (DMEM, PAA Laboratories) supplemented with 10% foetal calf serum (FCS). Cells were seeded into 96-well microtiter plates at the density 5,000 cells/0.1 ml/well, and 24 h after seeding, exposed in triplicate to serial dilutions (100 μM – 0.4 μM) of sample dissolved in dimethyl sulfoxide (DMSO). Controland blank wells were included in each plate. After 24 hand 72 h SRB assay was carried out. The cells were fixed in trichloroacetic acid (TCA) (25 μl of 50% w/v TCA perwell) for 1 h at 4°C, washed five times with distilled water, and stained with 50 μl of 0.4% SRB in 1% acetic acid for 30 min. The cells were washed five times with 1% aceticacid and air-dried. The stain was solubilized in 10 mMTRIS (pH 10.5) light absorption was measured using aplate reader (Thermo Labsystems) on 492 nm, with reference wavelength 690 nm. Cell cytotoxicity was expressed as a percentage of corresponding control value (nontreated cells) obtained in two independent experiments. The original data were analysed by a one-way ANOVA, followed by Duncan’s multiple-range post hoc test. Differences were considered significant at P < 0.05. The IC50values, defined as a dose of compound that inhibits cell growth by 50%, were calculated from concentration response curves.
3. Results and Discussion
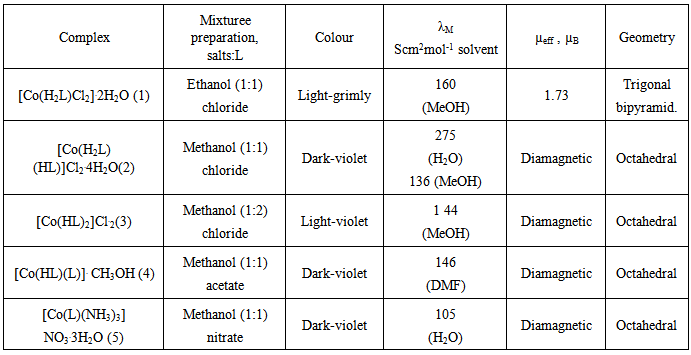
In reaction ITSCPL ligand with Co-salts (chloride, acetate and nitrate) we are received five Co complexes.With heating, solubility of salts were increased on account of complexation. For final number of oxidation state of metals, as on and shape of coordination ligand, the final influence have types of using salts in combination with pH of solution and nature of solvent (Table 1).Table 1. Physical-chemical characteristic of Co complexes
 |
| |
|
Accordingly, the reaction between CoCl2.6H2O/Co(NO3)2 and ligand ITSCPL in EtOH/MeOH solvent mixtureyielded mono (ligand) complex Co(II), [Co(H2L)Cl2].2H2O(1) and [Co(L)(NH3)3]NO3.3H2O(5), respectively. On the other hand, using only MeOH the reaction between Co(CH3COO)2. 4H2O and CoCl2.6H2O and the ligand resulted in the synthesis of bis-(ligand) complexes [Co(H2L)(HL)] Cl2.4H2O(2), [Co(HL)(L)]CH3.OH(4) and [Co(HL)2]Cl.2H2O(3). Complexes 2 and 4 are bis ligand complexes, despite the fact thata1:1 molar ratio of ligand and salt of was used .When the ratio was change to 1:2 in the favor of the ligand expected bis (lignad) complex [Co(HL)2]Cl.2H2O(3) was obtained. It is interesting to say that in the reaction of EtOH mixture containing CoCl2 and ligand ITSCPL, it did not result in oxidation of Co(II) to Co(III) (complex 1). For other complex (2, 3, 4 and 5) the oxidation occurred and we isolated cobalt (III) complexes. The oxidation is carried out using oxygen in the air according to the following reaction: 4Co(CH3COO)2+8H2L+O2→4[Co(HL)(L)]+8CH3COOH+2H2OIR spectrums (Table 2) showed characteristic values for dipole zwitter-ion. It is noticeable that more intense bands were found in the region above 3100 cm-1 which could be attributed to v(OH) vibrations of phenolic hydroxyl, in the protonated forms, the hydroxymethyl group and water molecules. Low energy bands originate from v(NH2), and v(NH+) vibrations respectively. IR spectroscopic proof of such pyridoxal structure, is confirmed by one or more bands around 2850 cm-1, which, besides ν(OH) also corresponds to ν(NH+) vibrations. Therefore, whenever the neutral form of ligand ITSCPL is coordinated in a complex (e.g. complexes 1, 2) a signal around 2850 cm-1is always clearly visible in the IR spectrum.Table 2. IR spectrums for Co complexes
 |
| |
|
Lastly, a large number of bands in the field of 1640-1400 cm-1 can be attributed to vibrations of heterocyclic ring, as well as δ(NH2) (probably about 1640 cm-1) and ν(C=N) chain(about 1550 cm-1). [13, 14]The values for magnetic moments were consistent with the coordination formula of the complex. Complex 1 contains an electronic d7Co(II) centre and, hence, paramagnetic behavior, which was confirmed by the expected value for the magnetic moment of 1.73μB.Other complexes of Co(III) are paramagnetic (d6 systems). The geometry of the complexes is estimated according to the measured characteristics of the complexes and the assumed coordination formulas.In complex 1 in coordination are the tridentate ONN ligand ITSCPL and two chlorine molecules, that indicating a trigonal-bipyramidal geometry (Figure 1).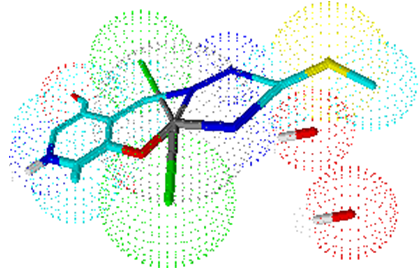 | Figure 1. Estimated geometry of complex 1 |
Complex 2 is a bis (ligand) complex, like complexes 3 and 4. Their central atom Co (III) is surrounded by six ligating atoms from two molecules of ligand ITSCPL.Coordination number six is arranged to octahedral geometry of the complex (Figures 2, 3 and 4).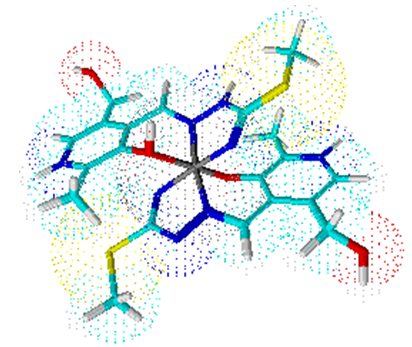 | Figure 2. Octahedral geometry of complex 2 |
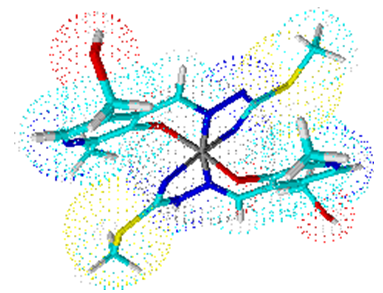 | Figure 3. Octahedral geometry of complex 3 |
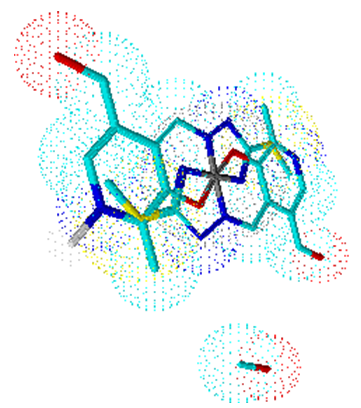 | Figure 4. Octahedral geometry of complex 4 |
In the case of mono (ligand) complex 5, the metal coordination sphere contains the ligand in its dianionic form and three molecules of ammonia (Figure 5).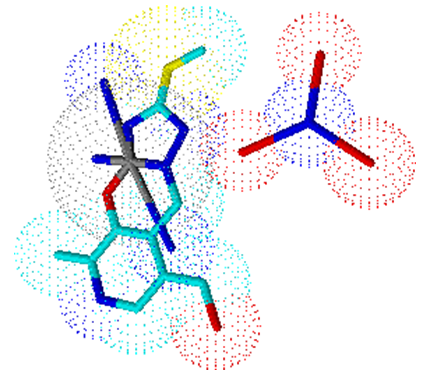 | Figure 5. Trigonal-bipiramidal geometry of complex 5 |
Cytotoxic effects of cobalt complexes were examined using SRB colorimetric assay, based on bonding of SRB compound with the total proteins of the living cells 23. Fig. 6. shows the IC50 values for the two cell linesand incubation times. The results suggest that cobalt complexes exhibit potent inhibitory action on MDA-MB-231 cell proliferation, where IC50 values for both incubation times were in the range of 3 μM. In MCF7 cell line cytotoxic effect is more pronounced after 72 h treatment, with IC50 value of 5 μM. Similarcytotoxic effects on MCF7 cell line were reported for nickel complexes of naphthaquinone thiosemicarbazone and semicarbazone, with IC50 values ranging from 2–4 μM23 [15].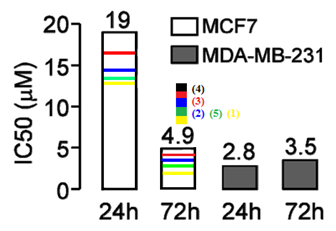 | Figure 6. Cytotoxic effects of cobalt complex on MCF7 and MDA-MB-231 human breast cancer cell lines after 24 h and 72 h of treatment |
4. Conclusions
As expected, the new synthesized cobalt complexes are interesting from the point of their structural features and biological activity. Moreover, obtained results clearly demonstrated that the series Co-complexes with pyridoxal-S-methylisothiosemicarbazone expressed strong inhibition of cell proliferation towards MCF7 and MDA-MB-231breast adenocarcinoma cell lines.
References
| [1] | M.C. Cardia, M. Begala, A. Delogu, E. Maccioni, A. Plumitalo, Farmaco (2000) 93. |
| [2] | M.J.M. Campbell, Coord. Chem. Rev. 15 (1975) 279. |
| [3] | S. Padhye, G.B. Kauffman, Coord. Chem. Rev. 63 (1985) 127. |
| [4] | R.B. Singh, H. Ishii, Cryt. Rev. Anal. Chem. 22 (1991) 381. |
| [5] | M.F. Belicchi, F. Bisceglie, C. Casoli, S. Durot, I. Morgenstern-Badarau, G. Pelosi, E. Pilotti, P. Tarasconi, J. Med. Chem. 48 (2005) 1671. |
| [6] | S. Novakovic, Z. Tomic, V. Jevtovic, V. Leovac, Acta Cryst C58 (2002) m358. |
| [7] | V. Jevtovic, Monograph, Synthesis, Physical-Chemical Characterisation, Structure and Biological Activity of Cu(II), Fe(III), Ni(II) and V(V) Complex with Pyridoxal Semi-, Thiosemi- and Isothiosemicarbazones, LAP Academic Publishing, Germany, 2010. |
| [8] | V.M. Leovac, V.S. Jevtović, L.J.S. Jovanovic, G.A. Bogdanović, J. Serb. Chem. Soc. 70 (2005) 393. |
| [9] | L.J. Jovanovic, V. Jevtovic, V. Leovac, L. Bjelica, J. Serb.Chem. Soc. 70 (2005) 187. |
| [10] | V. Leovac, V. Jevtovic, G. Bogdanovic, ActaCryst C58 (2002) m514. |
| [11] | V. Jevtovic, D. Vidovic, J. Chem. Crystallogr. (2010), 40(9), 794-798. |
| [12] | V.S. Jevtovic, Ph.D. Thesis, Faculty of Science, University of Novi Sad, 2002. |
| [13] | M. Ferrari Belicchi, G. Fava Gasparri, E. Leporati, C. Pelizzi, P. Taraskoni, G. Tosi, J.Chem. Soc. Dalton Trans., (1986) 2455. |
| [14] | M. Mohan, P.H. Madhuranath, A. Kumar, M. Kumar, N.K. Tha, Inorg. Chem., 28 (1989) 96. |
| [15] | Z. Afrasiabi, E Sinn, W. Lin, Y. Ma, C. Campana, S. Padhye, J. Inorg. Biochem.2005, 99, 1526–1531. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
