-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(4): 125-129
doi:10.5923/j.chemistry.20140404.03
Synthesis and Quantumchemical Study on Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 Complexes
Rumyana Yankova , Svetlana Genieva , Nenko Halachev , Ginka Dimitrova
Department of Inorganic and Analytical Chemistry, Assen Zlatarov University, Bourgas, Bulgaria
Correspondence to: Rumyana Yankova , Department of Inorganic and Analytical Chemistry, Assen Zlatarov University, Bourgas, Bulgaria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Using hydrothermal synthesis Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 complexes were obtained, which were characterized by X-ray and thermo-gravimetric analysis. The geometric and electronic structure parameters were calculated with the Density Functional Theory at the B3LYP level with 6-31G(d) basis set for H, O, S, Se and LANL2DZ for Hf in gas phase. The bond orders of the complexes were determined. HOMO-LUMO energies and calculated structures are shown. The key role in the LUMO play the d-atomic orbitals of Hf(IV). The data obtained for the selenate are reported for a first time.
Keywords: Hydrothermal synthesis, X-ray analysis, Density functional theory, Geometry optimization
Cite this paper: Rumyana Yankova , Svetlana Genieva , Nenko Halachev , Ginka Dimitrova , Synthesis and Quantumchemical Study on Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 Complexes, American Journal of Chemistry, Vol. 4 No. 4, 2014, pp. 125-129. doi: 10.5923/j.chemistry.20140404.03.
Article Outline
1. Introduction
- The elements of zirconium and hafnium form mixed crystals in nature because of their analogical chemical properties of their atoms. During decades different methods and technologies were developed for their separation with an aim to obtain hafnium which possesses the ability to absorb neutrons. This property occurs to be very useful for regulation of nuclear reactions [1, 2]. For the syntheses of different more complicated compounds of hafnium of certain interest are its simple salts because of the chemical sustainability of hafnium and its oxide HfO2. Different hafnium sulfates, which are isomorphous with the corresponding zirconium once, have been obtained by a reaction between HfO2 and sulfuric acid with concentrations in the range 50–90% and continuous evaporation of the solutions [3]. Their thermal stability was investigated and the intermediated products of destruction were defined. In general, the data concerning the structure and properties of the hafnium sulfates are scarce and not enough systematized [4-6]. Moreover, no data in the literature is found about the obtainment and characterization of hafnium selenites, salanates, and tellurates. The obtaining and the structure of Zr(SeO4)2∙4H2O [7] is reported only. Having in mind, the isostructure of the zirconium sulfate with this of hafnium, it can be expected that hafnium selenate may have analogical symmetry and similar crystalline-chemical characteristic with these of zirconium selenate.
2. Materials and Computational Methods
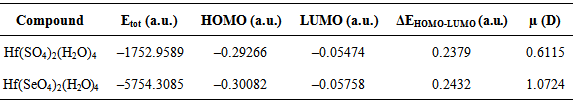
- Hafnium sulfate and hafnium selenate with a chemical composition Hf(XO4)2∙nH2O (X = S, Se) were obtained using the method of hydrothermal synthesis. The hydrothermal synthesis was carried out in a metal-teflonic autoclave with capacity 20 cm3 at 300°C for 6 hours as to 1 g HfO2 (Merck), 5 cm3 96-98% H2SO4 (Merck) and H2SeO4 (Merck) were added correspondingly. After the synthesis was over, the solid phase was washed with ethanol and dried at 100°C.The symmetry and the crystallographic parameters of the initial hafnium oxide, as well as the characterization of the obtained hafnium sulfate and selenate was determined using powder diffraction and thermal analyses. On the base of these analyses it is evident that the obtained substances are Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4.The quantum chemical calculations of the obtained compounds and HfO2 were realized by Becke's three-parameter hybrid functional, combined with the Lee-Yang-Parr – B3LYP [8] correlation function from the density functional theory basis set with added polarization functions of H, S, Se и O – 6-31G(d) and LANL2DZ for Hf, with the help of Gaussian03 [9]. Convergence criterion 10–8 a.u. for all calculations was used. The normal vibrations for each optimized molecule were calculated in order to investigate the nature of the reached stationary point in the optimization procedure. No imaginary frequencies for the structures were found, which means that they correspond to a status of minimum potential energy. To prepare the calculations and visualization of the results HyperChem 5.0 [10] and Molekel 5.4 program products [11] were used.
3. Results and Discussion
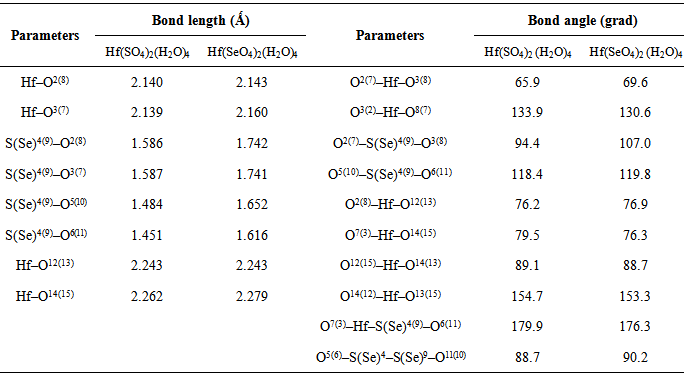
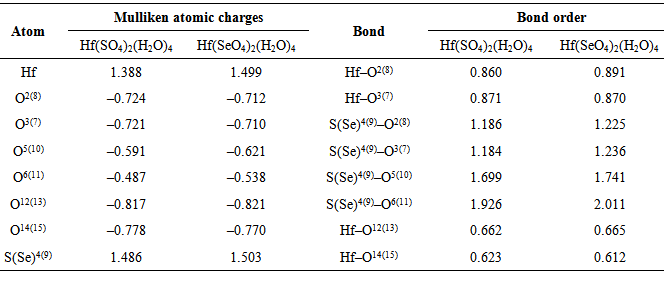
- The symmetry and the crystallographic parameters of the initial hafnium oxide, as well as the characterization of the obtained hafnium sulfate and selenate was determined using X-ray analysis [12]. The data obtained are shown in Fig. 1.The powder X-ray analysis shows that the used for hydrothermal synthesis hafnium dioxide possesses monoclinic symmetry (group P21/C) and parameters of the elementary cell: a = 5.1170Ǻ, b = 5.1754Ǻ, c = 5.2915Ǻ and α = γ = 90.00°, β = 99.216°. The obtained hafnium sulfate is a tetrahydrate with a composition Hf(SO4)2(H2O)4 and the results prove orthorhombic symmetry (group Fddd) and parameters а = 25.8700Ǻ, b = 5.5300Ǻ, c = 11.5900Ǻ, α = β = γ = 90.00°. The data from analogical investigations by other authors [1, 2] fully coincide with those obtained by us, which is evidence that the hydrothermal synthesis gives a tetrahydrate product. This product is isostructural with Zr(SO4)2(H2O)4.There is no data in the literature about X-ray analysis of hafnium selenates. Crystallographic parameters for Zr(SеO4)2(H2O)4 [7] are reported, which analogically with the sulfate should be isostructural with hafnium selenate. The experimental data show, that the newly synthesized selenate is with composition Hf(SeO4)2(H2O)4, possesses orthorhombic symmetry (group Fddd) and parameters of the elementary cell: a = 26.4900Ǻ, b = 5.6558Ǻ, c = 11.9489 Ǻ, α = β = γ = 90.00°. These data are analogous with the one for zirconium selenate [7]. From Fig. 1 and the obtained experimental data for the symmetry and crystallographic parameters is evident that the obtained Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 are isostructural. From the roentgenograms can be seen that both samples of the sulfate and selenate register peaks corresponding to HfO2, which has not reacted. The molecules of Hf(SO4)2(H2O)4 и Hf(SeO4)2(H2O)4 are visualized in Figure 2. The numbering of the atoms is given also. The results from the geometric optimization of the two molecules are given in Table 1.From the table can be seen that the values for the lengths of the bonds Hf–O and the angles O–Hf–O in the molecules of the two complexes are overlapping, while those for Se–O and O–Se–O are greater in comparison with those in the sulfur complex. It can be expected, that after dehydration of the complexes the waterless salt Hf(SeO4)2 will be thermally less stable than Hf(SO4)2.The electrostatic potential was calculated in order to investigate the reaction properties of the molecules with the chosen method of calculation [13].
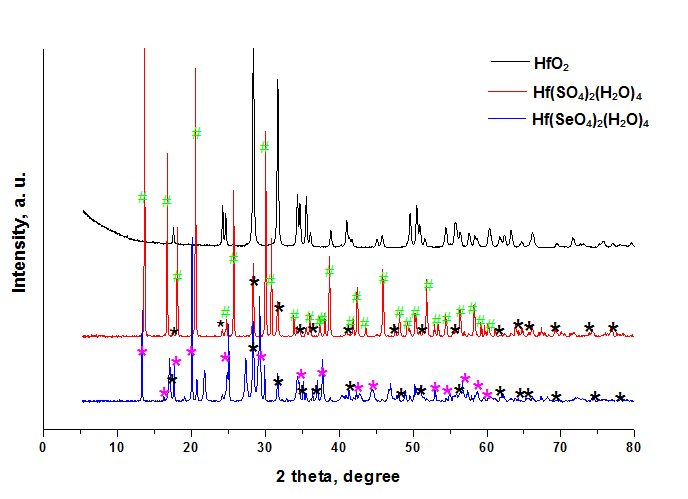 | Figure 1. X-ray analysis of *-HfO2, #-Hf(SO4)2(H2O)4 and *-Hf(SеO4)2(H2O)4 |
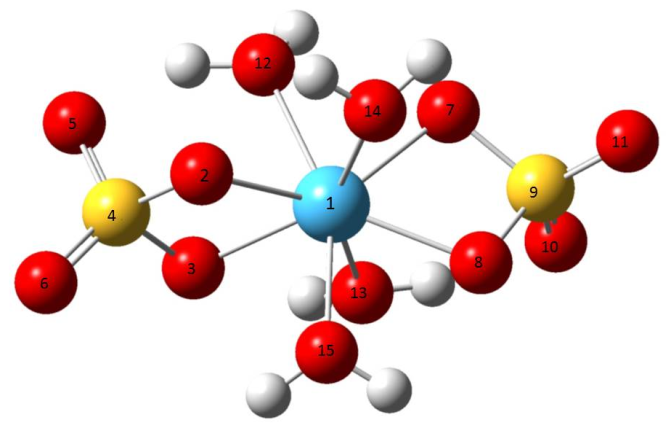 | Figure 2. Optimized geometrical structure and atomic labeling of Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4 (О-atoms are in red, Hf-atom is blue, S(Se)-atoms are in yellow) |
|
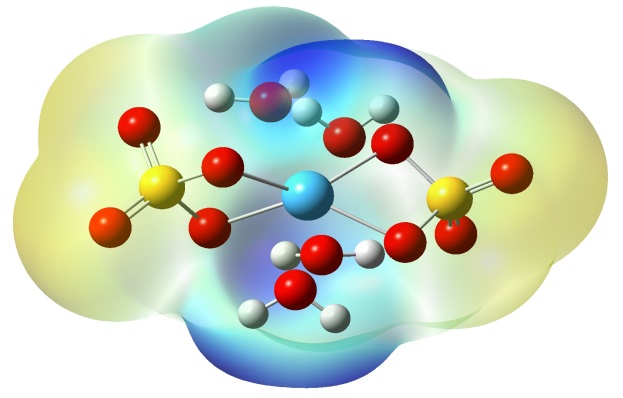 | Figure 3. Electrostatic potential on the surface of Hf(SO4)2(H2O)4 |
|
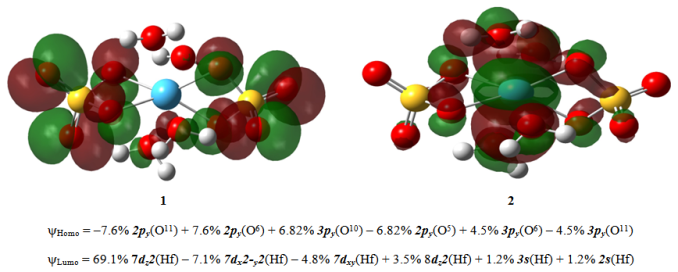 | Figure 4. Homo MO – (1) and Lumo МО – (2) of Hf(SO4)2(H2O)4 |
|
4. Conclusions
- Hafnium sulfate and hafnium selenate were synthesized using hydrothermal method. The substances were characterized by X-ray analysis and it was found that they are tetrahydrates – Hf(SO4)2(H2O)4 and Hf(SeO4)2(H2O)4. The crystallographic symmetry and the parameters of the elementary cell of the obtained substances and the initial reagent HfO2 were determined. The data for the sulfate totally coincide with the literature data and these for the hafnium selenate are reported for a first time. The geometry and the electronic structure, the net atomic charges according Mulliken and the order of the bonds in the obtained substances were calculated applying the density functional theory. The quantum chemical investigations are of certain interest for the chemistry of hafnium and its compounds which are not studied enough.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML