-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(4): 109-115
doi:10.5923/j.chemistry.20140404.01
Synthesis and Characterization of Pellicular γ-Zirconium Phosphate Fibrous Cerium Phosphate Nanocomposite Membranes
S. K. Shakshooki, S. R. EL-Tarhuni, A. M. EL-Hamady
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: S. K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Pellicular γ-zirconium phosphate, and nanosized fibrous cerium phosphate, γ-Zr.PO4 (HPO4).2H2O (pγ-ZrP), Ce(HPO4)2.2.9H2O (nCePf), respectively, were prepared and characterized by chemical, thermogravimtricanalysi(TGA), Fourier Transform IR spectrometer(FT-IR), Scanning electron microscopy(SEM) and transmission electron microscopy (TEM). Pellicular γ-zirconium phosphate- fibrous cerium phosphate nanocomposite membranes were obtained by mixing slurry aqueous solution of pγ-ZrP and nCePf at 45°C for 24h with stirring in wt/wt % ratio (67:33, 50:50 , 33:67 and 25:75%, (pγ-ZrP:nCePf) respectively. The resultant composites found to be: [γ-Zr.PO4.H2PO4.]0.6[Ce(HPO4)2].0.4,2.68H2O, [γ-Zr.PO4.H2PO4]0.51 [Ce(HPO4)2]0.49.3.56H2O, [γ-Zr.PO4.H2PO4.]0.34[Ce(HPO4)2]0.66.4.4H2O, [γ-Zr.PO4.H2PO4]0.27 [Ce(HPO4)2]0.73. 3.47H2O, respectively.The resultant composites were homogeneous thin films with good mechanical texture and thermal stability.
Keywords: Pellicular γ-zirconium phosphate, Fibrous cerium phosphate, Pellicular γ-zirconium phosphate-fibrous cerium phosphate nanocomposite membranes
Cite this paper: S. K. Shakshooki, S. R. EL-Tarhuni, A. M. EL-Hamady, Synthesis and Characterization of Pellicular γ-Zirconium Phosphate Fibrous Cerium Phosphate Nanocomposite Membranes, American Journal of Chemistry, Vol. 4 No. 4, 2014, pp. 109-115. doi: 10.5923/j.chemistry.20140404.01.
Article Outline
1. Introduction
- Metal phosphates chemistry recently has attracted attention in order to obtain materials with wide range of applications. Among these tetravalent metal phosphate which is very insoluble compounds. Other than amorphous [1], they exist in two dimensional (2-D) and three dimensional (3-D) structures [2].Porous layered MIV phosphates with α, and -structures [3-5], α-MIV(HPO4)2. H2O,-MIV(HPO4)2.5H2O and -MIV. PO4.H2PO4. 2H2O, are well known compounds, (where M = Ti, Zr, Hf, Sn, Ce…..). These materials contain structural POH groups with labile protons and therefore potential conductance [6, 7], solid acid catalysts [8] and intercalates [9].A number of organic membranes are available today. However, very little studies were carried out on inorganic membranes of tetravalent metals. Membranes consists of inorganic polymers such as membrane of MIV phosphates are very attractive and could suitable for many processes of chemical technology such as waste disposal of metal ions, intercalates electrical conductance and solid acid catalysts.Fibrous cerium phosphate has been reported [10]. Pellicular zirconium phosphate, Zr(HPO4)2. H2O and pellicular hafnium phosphate, Hf(HPO4)2. H2O were reported [11, 12]. The pellicular membranes are two dimensional (2-D) structure membranes.In our laboratory we under taking systematic studies on different varieties of pellicular membranes with general formulae, MIV (HPO4)2. H2O [11, 13], MIV. PO4.H2PO4. 2H2O [14] and ZrxTi(1-x)(HPO4)2. H2O [15] that were prepared by different methods.Recent years have seen upsurge interest in the study of inorganic solid-solid and inorganic solid-organic polymer composite materials. The resultant composite material usually does not have either characteristic of original materials and can be considered as materials with almost new physical and chemical properties [16].Inorganic membranes-membrane composites are not recent invention, examples are SiO2-TiO2, SiO2-ZrO2, Pd-Al2O3, Pd thin filmdiposited on ceramic materials …etc [17, 18].However, to date there are no reports about membrane-membrane composite materials of MIV phosphates except the one reported by us [19] Here we are reporting the synthesis and characterization of novel pellicular -zirconium phosphate-fibrous cerium phosphate nanocomposite membranes.
2. Materials and Methods
- Chemicals:ZrOCl2.H2O, CeSO4.4H2O, H3PO4(85%) of BDH, HF (40%) of Reidel De-Haen. Other chemicals used were of analytical grade.Instruments used for characterizationX-Ray powder diffractometer Siemens D-500, using Ni-filtered CuKα (λ = 1.54056Å), TG/DTA SIIExtra6000 TGA Perkin Elmerthermogravimetric analyzer(TGA)US, Fourier Transform IR spectrometer, model IFS 25 FTIR Bruker, Scanning electron microscopy (SEM) Jeol SMJ Sm 5610 LV, Transmission electron microscopy (TEM) Zeiss TEM 10CR, pH Meter WGW 52.Preparation of fibrous cerium phosphate 100 ml 0.05M CeSO4.4H2O in 0.5M H2SO4 were added drop wise to 100ml 6M H3PO4 at 80°C with stirring. The stirring was continued at that temperature for 4h. The resultant product was dispersed in 2 litre distilled water with stirring for 1h. Then subjected to washing by distilled water up to pH~3.5, then kept in 2 liter distilled water to be, as such, in slurry aqueous form.Preparation of pellicular - zirconium phosphate membrane-zirconium phosphate monoammonium form , -Zr.PO4. NH4HPO4, was obtained by reacting 50ml of ZrOCl2.H2O (1.3M in 8M HF) with 450ml of 2M NH4H2PO4 in Pyrex round flask at 80°C with stirring for 24h. The resultant product was filtered washed with distilled water up to pH~3, filtered and air dried. The ammonium form was converted to hydrogen form using 1MHCl with vigorous stirring at 15°C for 24h. Flexible thin films, the pellicular -Zr.PO4. H2PO4.2H2O (p-ZrP) membrane. The minor product (~10%) found to be crystalline -zirconium phosphate. (for every 2g of-Zr.PO4.NH4HPO4 400ml 1M HCl were required).Preparation of pellicular - zirconium phosphate-fibrous cerium phosphate nanocomposite membranesSlurry aqueous solution of 0.3g of pellicular -zirconium phosphate (p-ZrP) in 150ml distilled water was added t gradually to slurry aqueous solution of 0.3g fibrous cerium in300ml distilled water at 45°C, with stirring , the stirring was continued after complete addition and at 45°C for 24h. The resulted product was filtered on filter paper using Buchnner funnel , washed with distilled water (50ml) twice then left to dry in air, the resulted product was homogeneous a thin films, with [γ-Zr.PO4.H2PO4]0.51[Ce(HPO4)2]0.49. 3.56H2O.In similar manner, using the above experimental parameters, composite pellicular -zirconium phosphate- fibrous cerium phosphate membranes: [γ-Zr.PO4.H2PO4.]0.6 [Ce(HPO4)2].0.4,2.68H2O, [γ-Zr.PO4.H2PO4.]0.34[Ce(HPO4)2]0.66.4.4H2O and [γ-Zr.PO4.H2PO4]0.27 [Ce(HPO4)2]0.73. 3.47H2O, were obtained by mixing slurry aqueous solution in wt/wt % ratio, 60:40, 33:67 and 25:75 of p-ZrP:nCePf, where the mixing weights ratio are 0.15g:0.1g, 0.15:0.3g and 0.1:0.3g, respectively. After complete mixing of the above slurry aqueous solutions, at 45°C the resultant products were filtered on filter paper using Buckner funnel, washed with distilled water and dried in air. They were homogeneous flexible thin films.
3. Results and Discussion
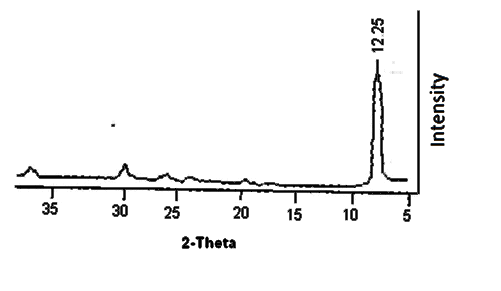
- Nanosized cerium phosphate membrane, Ce(HPO4)2. 2,9H2O(nCePf) ,was prepared and characterized by chemical, XRD, thermal analysis FT-IR, SEM and TEM. X-ray diffractogram (XRD) of fibrous cerium phosphate membrane is shown in Figure 1 with d001= 10.85Å.
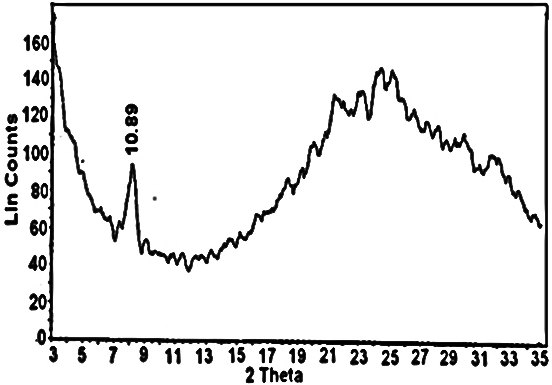 | Figure 1. XRD of fibrous cerium phosphate |
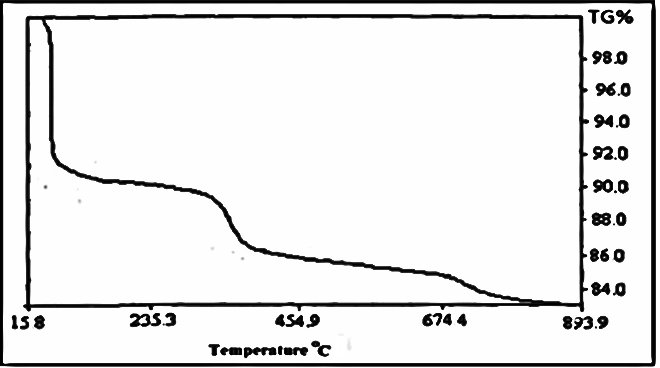
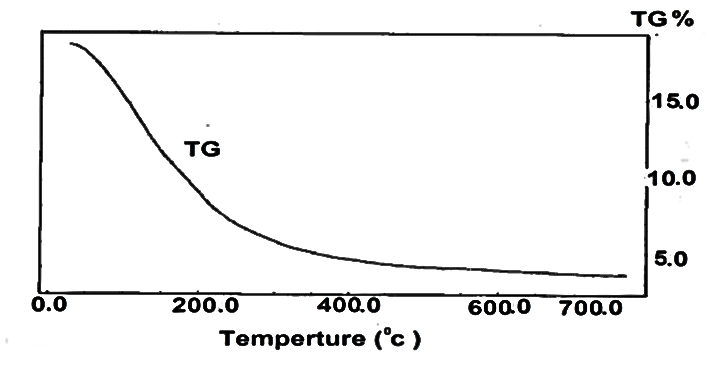 | Figure 2. TG of fibrous cerium phosphate |
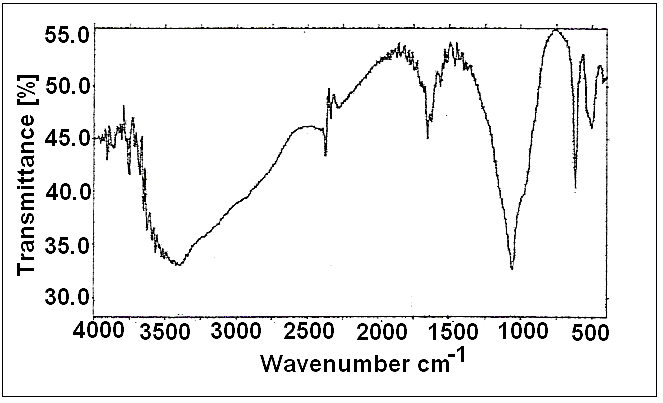 | Figure 3. FT-IR spectrum of fibrous cerium Phosphate |
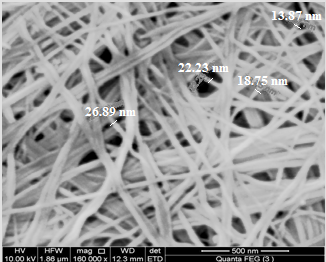 | Figure 4. SEM image of fibrous cerium phosphate |
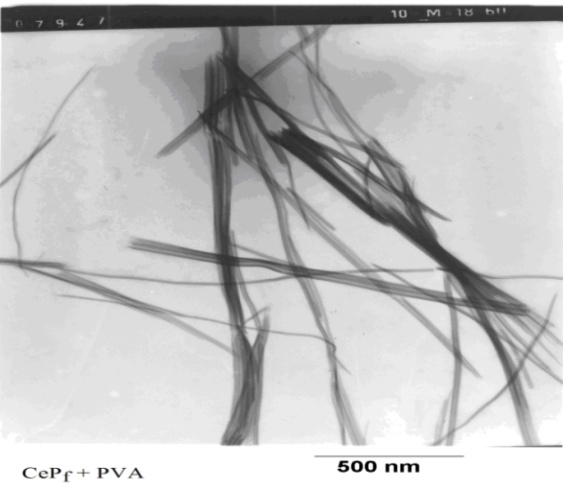 | Figure 5. TEM image of fibrous cerium phosphate |
 | Figure 6. XRD of pellicular -zirconium phosphate, showsiso-oriented structure of its crystalline form |
 | Figure 7. TG of -zirconium phosphate |
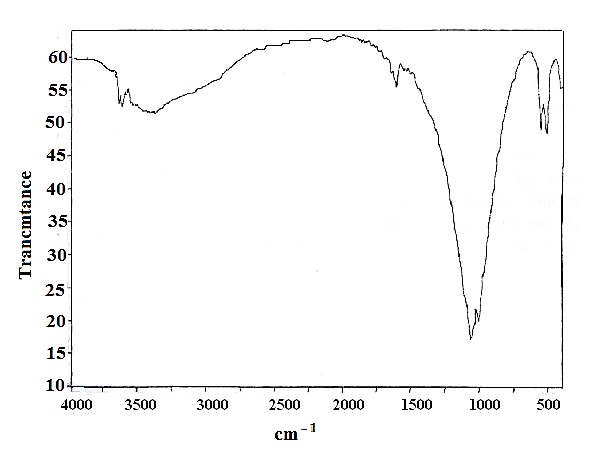 | Figure 8. FT-IR spectrum of pellicular -zirconium phosphate |
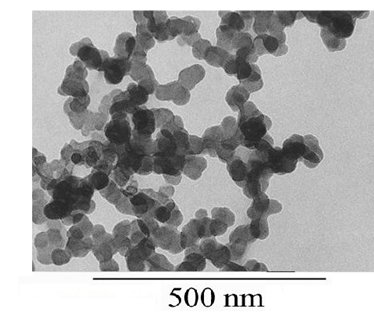 | Figure 9. TEM image of -zirconium phosphate |
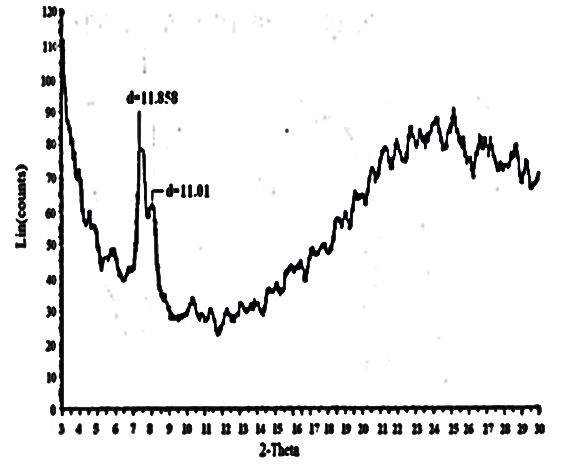 | Figure 10. XRD of [γ-Zr.PO4.H2PO4.]0.6[Ce(HPO4)2]. 0.4,2.68H2O |
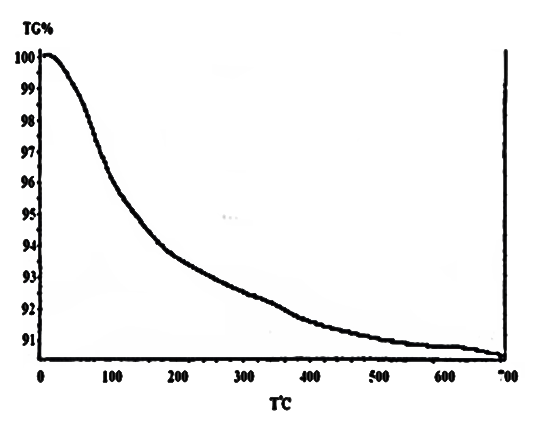 | Figure 11. TG of [γ-Zr.PO4.H2PO4.]0.6[Ce(HPO4)2]. 0.4,2.68H2O |
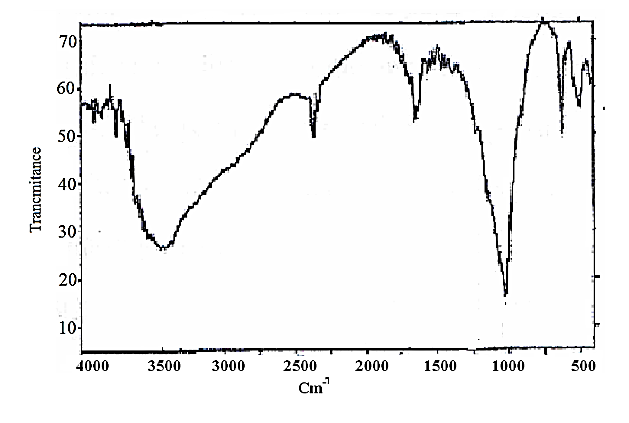 | Figure 12. FT-IR spectra of [γ-Zr.PO4.H2PO4.]0.6[Ce(HPO4)2]. 0.4,2.68.H2O |
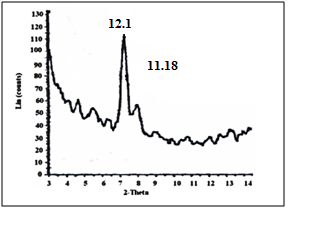 | Figure 13. XRD of [γ-Zr.PO4.H2PO4. ]0.51'[Ce(HPO4)2. ]0.49 . 3.56H2O |
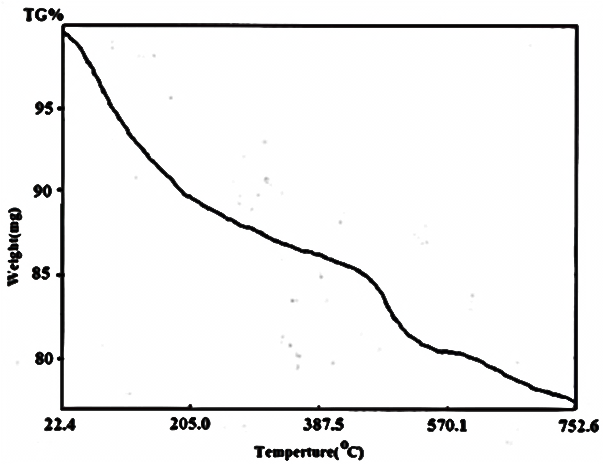 | Figure 14. TG of [γ-Zr.PO4.H2PO4. ]0.51'[Ce(HPO4)2.]0.49 . 3.56H2O |
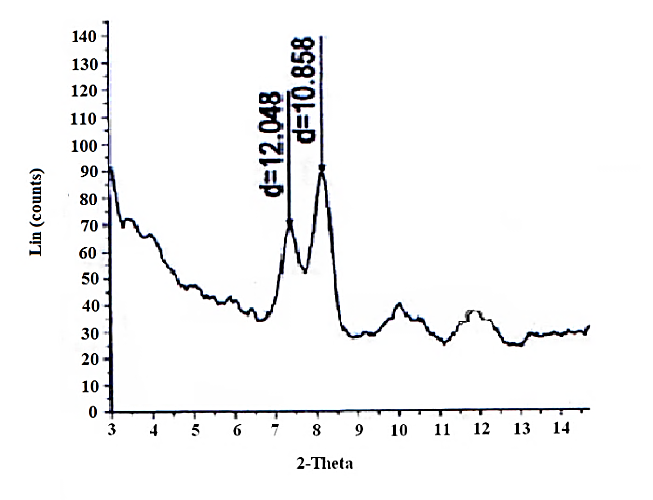 | Figure 15. XRD of [γ-Zr.PO4.H2PO4. ]0.34[Ce(HPO4)2 ]0.66 . 4.4 H2O |
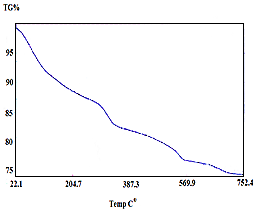 | Figure 16. TG of [γ-Zr.PO4.H2PO4.1.4H2O]0.34 [Ce(HPO4)2 ]0.66.4.4 H2O |
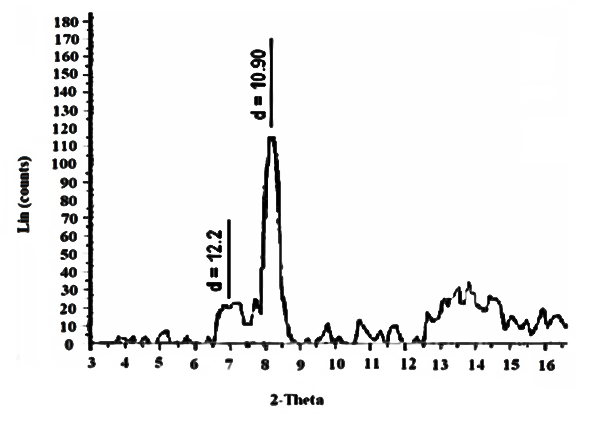 | Figure17. XRD of [γ-Zr.PO4.H2PO4]0.27[Ce(HPO4)]0.73,3.47H2O |
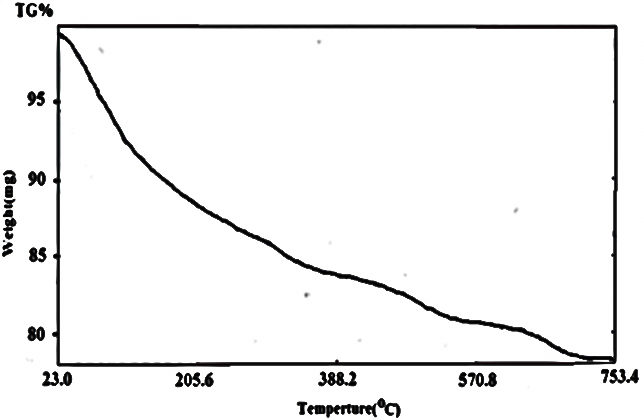 | Figure 18. TG of [γ- Zr.PO4.H2PO4. ]0.27[Ce(HPO4)2]0.733.47H2O |
4. Conclusions
- Pellicular γ-zirconium phosphate, and nanosized fibrous cerium phosphate, γ-Zr.PO4 (HPO4).2H2O (pγ-ZrP), Ce(HPO4)2. 3H2O (nCePf), respectively, were prepared and characterized. The study shows that metal(IV)phosphate nano composite membranes can be obtained by mixing slurry aqueous solution of their membranes in required wt/wt% mixing ratios. The resultant composite compounds were flexible homogeneous thin films. XRD of composites show that it is possible to obtain tailored made inorganic membrane-membrane composites, where their XRD patterns shows two d spacing reflections which are related to the d spacing reflection of their parent compounds. The XRD retain the d spacing of their parent materials FT-IR spectra of every composite materials are very similar to the FT-IR spectra of their original materials. These novel composite membranes can be considered for potential applications as solid acid catalysts, inorganic ion exchangers intercalates and as proton conductance materials.
AKNOWLEGEMENTS
- To Department of Chemistry, Faculty of Science, Tripoli University, for providing facilities for this research, and to Geol. Adel Bayuomi, Institute of Mineral Resources, Cairo, Egypt for providing facilities for TGA, SEM and TEM analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML