-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(3): 101-108
doi:10.5923/j.chemistry.20140403.03
Some Heavy Metals Content of Cabbage and Soil Cultivated in the Bezi Bar Farm Area of Katima Mulilo, Namibia
J. Abah1, P. Mashebe1, S. T. Ubwa2, D. D. Denuga1
1Department of Mathematics, Science and Sport Education, Faculty of Education, University of Namibia, Katima Mulilo Campus, Private Bag, 1096, Katima Mulilo, Namibia
2Department of Chemistry, Faculty of Science, Benue State University, P.M.B. 1026, Makurdi, Nigeria
Correspondence to: J. Abah, Department of Mathematics, Science and Sport Education, Faculty of Education, University of Namibia, Katima Mulilo Campus, Private Bag, 1096, Katima Mulilo, Namibia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The accumulation of heavy metals in agricultural soils is of increasing concern due to food safety and potential health risks. This study determined the concentrations of chromium, cadmium, cobalt, lead, nickel and arsenic in the cabbage and soil obtained from the Bezi bar farm area of Katima Mulilo and compare the results with regulatory limits. Samples of the cabbage and soil were randomly collected from the same points in the farm during mid-October, 2013; 4 Months After Planting (4MAP). The samples were digested using EPA methods 3052 (cabbage) and 3050B (soil) followed by ICP-OES (ICP: Perkin Elmer Optima 7000 DV) analyses. The heavy metals concentrations in the cabbage varied between 2.00 mg/kg - 0.05 mg/kg while in the farm soil, the result varied between 3.60 mg/kg - 0.2 mg/kg. The soil-cabbage transfer factors of the heavy metals showed the following bio-accumulation trend: Ni (1.25) > Cd (0.40) > Pb (0.23) > Cr (0.20) > Co (0.04) > As (0.03). There were very high positive correlations between the cabbage and soil heavy metals which suggest common source of input. Although, the present levels of the heavy metals were lower than their regulatory limits in vegetable and agricultural soil; continuous accumulation is a concern to the produce safety and quality because of chronic human exposure. The soil properties revealed poor nutrient status resulting to the high usage of chemical fertilizer in order to improve cabbage yield and this pose threat to the produce quality and safety. Therefore, it becomes imperative for the farmer to consider using organic manure in enhancing the soil fertility in order to limit accumulations of residual chemicals in the soil and crop.
Keywords: Cabbage, Chemical fertilizer, Heavy metals, Soil fertility
Cite this paper: J. Abah, P. Mashebe, S. T. Ubwa, D. D. Denuga, Some Heavy Metals Content of Cabbage and Soil Cultivated in the Bezi Bar Farm Area of Katima Mulilo, Namibia, American Journal of Chemistry, Vol. 4 No. 3, 2014, pp. 101-108. doi: 10.5923/j.chemistry.20140403.03.
Article Outline
1. Introduction
- Pollutant indicators such as heavy metals are ubiquitous in the environment as a result of both natural and anthropogenic activities, and their excess concentrations in farm soils threaten the qualities of crop produces. Heavy metals are closely connected with deterioration of the environment and life quality and chronic exposure to low level of heavy metals can lead to severe health effects which in excess could result in acute poisoning [1]. Following the challenge of depleting soil nutrients most famers are facing, there have been increasing usages of chemical fertilizers and organic manures as means of augmenting soil fertility to improve yield. The increasing usage of agrochemicals in crop cultivations has resulted in the accumulation of excess nutrient elements in the rooting and deeper soil zones [2]. Land contamination/degradation is a threat to sustainable agricultural development and food security in the developing countries [3]. Of particular concern, the use of chemical fertilizer is done without proper advice from agricultural officers [4, 5] and the misuse including over- and under-dosage as well as mixing of different fertilizers may have unintended impacts on the crop production and environment [6]. Some potentially toxic metals and trace elements present in agricultural soils enter the human body through the food chain [1] and this could become a significant source of health concern following continued absorption and bio-accumulation. Cabbage is about the most highly cultivated and consumed leafy vegetable in Katima Mulilo and could thus, represent an important source of trace metals in human diets in this area. Generally, Vegetables are common diet taken by various human populations throughout the world due to their richness in vitamins, minerals, fibers and anti-oxidative effects [7]. However, vegetables cultivated on contaminated soils take up heavy metals in quantities large enough to cause potential health risks to the consumers [8-11, 12]. Leafy vegetables such as amaranth and cabbage have particularly been reported to be good absorber of heavy metals from soil [13-15]. Risks due to chemical contaminants in the terrestrial environment are often assessed by comparing current concentrations against reference concentrations above which adverse effects are considered likely to occur [1]. The health impacts of agricultural chemicals used in crop production are a function of their degree of accumulation in environmental sinks: soil, air, water, plants and the degree to which humans are exposed to them [16]. While increased agriculture and agrochemical uses are generally considered as panacea for farmers to increase production [1]; the farming practices and uses of agrochemicals including possible environmental pollution from agriculture in Katima Mulio, Namibia have not been investigated. Thus, it is envisaged that the baseline information presented in this study will contribute to the understanding of agricultural situation in Namibia and the possible effects of the use of chemical fertilizer in cabbage cultivations. This may be useful for sustainable agriculture, identify training need for appropriate and healthy crop production and limit consumers’ exposure to dietary chemical contaminants.
2. Materials and Method
2.1. Study Area
- This study was conducted in Katima Mulilo located on latitude 17°30′00″S and longitude 24°16′00″E based on the World Geodetic System (WGS) 84 coordinate reference system. Katima Mulilo is the capital of Zambezi Region, Namibia's far northeast extension into central Southern Africa (Figure 1). The region is more tropical than the rest of Namibia, with a higher rainfall (average annual rainfall varied between 550mm and 700mm) and warmer winters [17]. However, the rainfall pattern can be very variable and most rain falls in the summer, peaking in January and February. Cabbage cultivations in the Bezi bar farming area normally take place during winter season (May to October) and is sustained by irrigation agriculture, using river Zambezi as the source of water. Following years of continous cultivations, the farm soils are now warn out and the farmers relied heavily on soil improvement via chemical fertilizers application to optimize yield. The cabbage; STAS 3315 hybrid variety (white cultivar) was cultivated with fertilizer (NPK 2:3:2) applications at 7 Days After Planting (DAP), 15 DAP, 28 DAP and 48 DAP before maturity in an average of 120 DAP.
 | Figure 1. Map of Namibia showing the location of Katima Mulilo in the far northeast extension |
2.2. Cabbage Sample Collection and Pretreatment
- A total of five densely leaved heads of the cabbage; about 4 Months After Planting (4MAP) were randomly harvested in mid-October, 2013 from the farm. In each sample, all the damaged outer leaves were removed as shown in figure 2 below and the rest washed with double distilled water to remove adhered airborne and soil particles before conveying to the laboratory for further processing and analysis.
 | Figure 2. STAS 3315 hybrid variety (White cabbage cultivar) harvested 4MAP |
2.3. Cabbage Sample Preparation and Digestion
- The densely leaved heads of the cabbage were shredded and homogenized after which three different measures of 10.0 g each were oven dried at 80°C to constant weight. Each of the dried samples were ground, passed through a 2 mm sieve mesh size and 0.5 g of the powder taken for microwave acid digestion using EPA method 3052. Each 0.5 g sample was transferred into separate Teflon digestion vessel followed by the addition of 9.0 mL concentrated nitric acid plus 3 mL concentrated hydrofluoric acid in a fume hood. Also, 1 mL of 30% hydrogen peroxide was added to each vessel contents to aid complete oxidation of the sample [18] after which they were allowed to react for approximately one minute before capping and sealing the vessels. The vessels were then placed in a rotor and heated in laboratory-grade microwave equipment with the temperature rising gradually to 180℃ over the first 6 minutes after which the heating was continued further at the 180℃ for 9.5 minutes. The vessels were allowed to cool to room temperature, removed and opened in a fume hood and the content filtered through Whatman No. 41 filter paper into a 100 mL volumetric flask after which the volume made up to the mark with double-deionized water.
2.4. Sample Analysis
- A multi-elemental standard solution of 1000 mgkg-1 containing all the analysed elements (Pb, Cr, Cd, Co, As and Ni) was used for instrument calibration. Ten (10) mL of each filtrate (cabbage digestate) was taken and mixed with equal volume of matrix modifier and then analyzed using ICP-OES (ICP: Perkin Elmer Optima 7000 DV) for the levels of lead, chromium, cadmium, arsenic, cobalt and nickel.
2.5. Soil Samples Collection and Preparation for Physico-chemical Analysis
2.5.1. Heavy Metals
- Five soil samples were randomly collected at the point where the cabbage samples were collected. The soils were air dried, crushed, passed through a 2 mm mesh sieve and digested according according to EPA method 3050B for Inductively Coupled Plasma-Optical Emission Spectrophometer (ICP-OES) [19].
2.5.2. Farm Soil Properties
- The five soil samples were air-dried at room temperature, bulked and crushed, then, passed through a 2 mm mesh sieve. The farm soil pH and conductivity were measured in a soil/water slurry (1:2.5) using electrometric method, total nitrogen was determined by modified Kjeldahl method (ISO 11261), extractable phosphorus was determined by the Olsen method [20], organic carbon was determined by the dichromate oxidation method [21], while organic matter was calculated from the result of organic carbon using a multiplication factor of 1.724, and the exchangeable cations: sodium, potassium, magnesium and calcium were extracted using 1N ammonium acetate at pH 7.0 [22], followed by ICP-OES analysis.
2.6. Data Handling
- Data generated from triplicate analyses were calculated as mean of the heavy metal concentrations in the farm soil and cabbage. Furthermore, soil-cabbage transfer factors were computed as well as inter-elemental correlation between the cabbage and farm soil mean metal concentrations to access the degree of correlation between the soil and cabbage metals content.
3. Results and Discussion
3.1. Concentrations of the Heavy Metals in the Cabbage
- The results in Figure 3 show the concentrations (ppm) of the heavy metals in the cabbage. Ni (2.00 mg/kg) recorded the highest concentration while Co (0.05 mg/kg) recorded the lowest level. The results of the other metals showed 0.60 mg/kg Cr, 0.08 mg/kg Cd, 0.30 mg/kg Pb and 0.10 mg/kg As. Although, these concentrations were lower than the reported health regulatory limits of the heavy metals for ingested vegetables [23] Appendix 1; human bio-accumulations following prolonged ingestions could pose adverse health challenge. No regulatory limit was recorded for cobalt because it has been reported that both field and laboratory investigations indicated a lack of biomagnification of cobalt in food webs [24, 25]. The report also submitted that the bioaccumulation potential of cobalt in natural ecosystems is not high and therefore, despite meeting the criteria for persistence, the element do not meet the criteria for bioaccumulation as set out in the persistence and bioaccumulation regulations [24]. Research findings revealed that the period since 1973 has been so notable for the increase in the awareness that anomalies in trace elements supply can influence human health and wellbeing without necessarily producing diagnostically specific clinical changes [26]. In addition, it is now recognized that the clinical expression of a latent deficiency or excess of trace metals is often contingent on variables such as enhanced growth, malnutrition and challenges such as stress, infection or injury. It was also emphasized that the ability of specific population to tolerate such challenges can be influenced by anomalies in trace element status and hence, the need to maintain intake within tolerable limits [26]. The intake of metal ions via many food components, can be a double edged sword [25] because ev7n the known metals and metalloids considered as essential, can become toxic to the health of the consumer if present in excess of body requirement. For the maintenance of health, great deals of preventative measures are being put in place to minimize the ingestion of potentially toxic metal ions via food items. For instance, from monitoring endogenous levels of metal ions in foods and drinks to detecting contamination during food preparation, the European countries spend significant resources to avoid metal intake by the general population [28, 29]. Chemical elements present in human foods are readily ionized and ultimately get absorbed completely by the body. Absorption of these minerals is enhanced at low concentration of fiber, and in the absence of phytates and oxalates in the diet [29]. Beyond radicals, metal ions can disrupt normal cell and tissue function through multiple pathways including interactions with proteins and other biomolecules and disruption of membrane potentials [27].
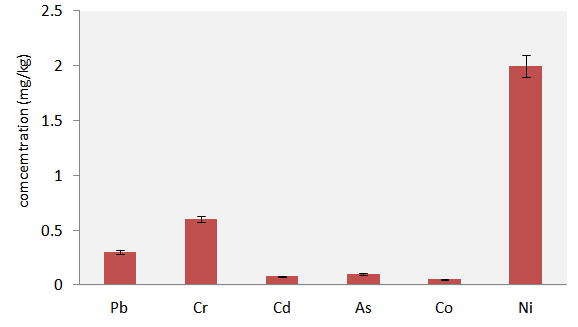 | Figure 3. Concentrations (mg/kg) of the heavy metals in the cabbage head |
3.2. Soil Heavy Metals Concentration of the Cabbage Farm
- Figure 4 presents results of the heavy metals concentration (mg/kg) in the cabbage farm soil. Arsenic (As) recorded the highest mean concentration of 3.60 mg/kg while Cd (0.2 mg/kg) recorded the lowest mean level. The other results showed Pb mean level of 1.10 mg/kg, Cr 3.0 mg/kg, Co 1.30 mg/kg and Ni 1.6 mg/kg. Although, these results were lower than the Canadian soil quality guidelines of the heavy metals for protection of environmental and human health [30] (Appendix 1); metals accumulation in agricultural soils is of increasing concern due to food safety, leaching into underground water, surface run-off to nearby rivers and the potential health risks. Potentially harmful metals in soil may come from the soil bedrock itself and anthropogenic sources like solid or liquid waste deposits, agricultural inputs and fallout of industrial and urban emissions [31]. In this study, the main sources of the heavy metals may be the soil bedrock and agricultural inputs (chemical fertilizers). Soil heavy metals are accumulative because of their non-degradability nature. In excess concentration, some heavy metals are phytotoxic to crops. For example, research findings have shown that plants grown on soil containing high levels of Cd show visible symptoms of injury reflected in terms of chlorosis, growth inhibition, browning of root tips and death [32, 33]. In another report, chromium stress was said to induce three possible types of metabolic modification in plants: (i) alteration in the production of pigments, which are involved in the life sustenance of plants (e.g., chlorophyll, anthocyanin) [34]; (ii) increased production of metabolites (e.g., glutathione, ascorbic acid) as a direct response to Cr stress, which may cause damage to the plants [35] and (iii) alterations in the metabolic pool to channelise the production of new biochemically related metabolites, which may confer resistance or tolerance to Cr stress (e.g., phytochelatins, histidine) [36]. Pb was also reported to exert adverse effect on morphology, growth and photosynthetic processes of plants [37]. High level of soil Co was observe to affect the translocation of P, S, Mn, Zn and Cu from roots to tops in cauliflower while Plants grown in high Ni2+ containing soil showed impairment of nutrient balance and resulted in disorder of cell membrane functions [37].
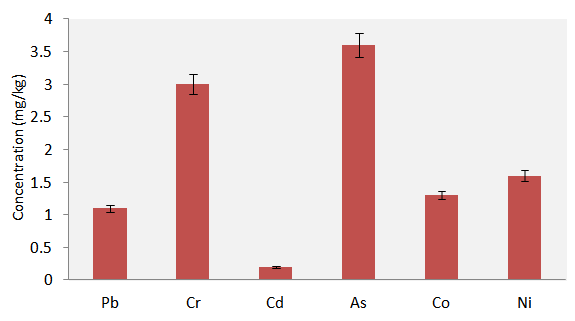 | Figure 4. Concentrations (mg/kg) of the heavy metals in the farm soil |
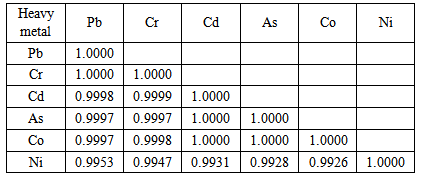
3.3. Inter-elemental Correlation Analysis of the Heavy Metals
- The result of the inter-elemental correlation analysis between the cabbage and farm soil heavy metals (Table 1) showed very high positive correlations. This suggest common source of the metals input and also implies that as the soil receives high load of the heavy metals via anthropogenic sources, the accumulated metals are absorbed by the cabbage grown on the soil and bioaccumulate into tissues. The possible sources of heavy metals input in the cabbage farm include the soil bedrock, aerial deposition, usage of chemical fertilizers and pesticides as well as the irrigation water. At present, the frequent application of chemical fertilizer; up to four times within a single cropping cycle coupled with 100% irrigation agriculture used to cultivate the cabbage may have significant implication for soil accumulation of heavy metals and bio-accumulation by the crop into tissue. Research work has shown that increasing agricultural intensification, such as the increasing inputs of agricultural machinery, fertilizer, pesticide, and irrigation water, as well as the climatic and environmental changes may be posing threats to the local soil and crop quality [38]. However, the lack of investigation into contaminants in the agricultural soils and crops derived from different types of agricultural activities in the study area makes it difficult to identify potential problems from certain agricultural practices.
|
3.4. Soil-cabbage Transfer Factors of the Heavy Metals
- The soil-cabbage transfer factors of the heavy metals (Figure 5) showed the following bio-accumulation trend: Ni (1.25) > Cd (0.40) > Pb (0.23) > Cr (0.20) > Co (0.04) > As (0.03). Accumulation of Heavy metals in vegetables occur by various sources but soil is considered the major one [39]. The soil- plant transfer process constitutes the major path way of human exposure to soil metals contamination. Following prolong ingestion, the elevated level of Ni absorption and bio-accumulation by the cabbage could become a source of health concern due to the classification of the metal as potential human carcinogen. Apart from Ni, it was observed in this study that cabbage showed general potential of absorbing the metals from soil and bio-accumulating them into its dense leaf tissues. Thus, continous human exposure to the heavy metals via ingestion of the cabbage is likely as the crop is a highly valued vegetable widely cultivated and consumed in the study area. This therefore, presents concern to the product quality and safety for human consumption because of the reported health effects when threshold limits of the identified metals are exceeded following prolonged food ingestion.
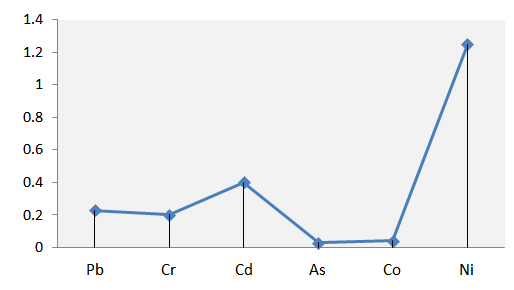 | Figure 5. Transfer factors of the heavy metals in the cabbage |
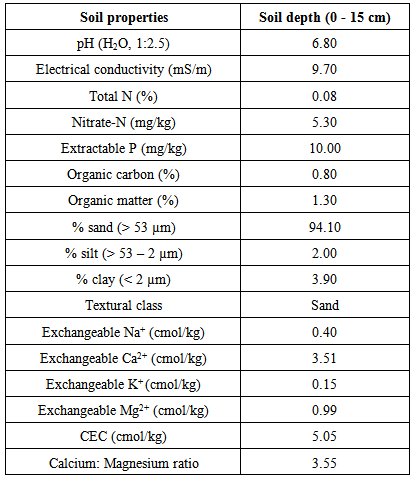
3.5. Soil Properties of the Cabbage Farm
- The soil properties of the cabbage farm (Table 2) showed that the soil recorded a pH of 6.9 which indicates neutral soil that enables almost all varieties of garden plants to grow and sustain. However, in a production guideline for cabbage, the South Africa’s Department of Agriculture, Forestry and Fisheries recommended soil pH of 5.5 – 6.5 [40]. It must be noted that soil pH can change throughout the year due to factors including rain type, agronomic practices and depletion of certain nutrients among others. The electrical conductivity of the farm soil saturation extract (ECe) recorded 0.10 dS/m. Conductivity is the measure of soluble salts (salinity) in a soil. An ECe value of 0.00 – 0.25 dS/m indicates probable nutrient elements deficiency within the soil zone. Other results of the soil properties showed that the the cabbage farm recorded low total nitrogen (0.08%) and low Nitrate-N (5.30 ppm), moderate levels of organic matter (1.30%), very low extractable phosphorus (10 ppm) for vegeatble crop. The cation exchange capacity of the soil (5.05 cmol/kg) was low and this suggest probable leaching of nutrient elements: potassium, calcium and magnesium beyond the surface soil. The poor nutrient status of the cabbage farm informed the high usage of chemical fertilizer by the farmer which portends challenge to the product safety and quality.
|
4. Conclusions
- The results of this study revealed varying concentrations of chromium, cadmium, cobalt, lead, nickel and arsenic in the cabbage and soil obtained from the Bezi bar farm area of Katima Mulilo, Namibia. Although, the present levels of the heavy metals were lower than their regulatory limits in vegetable and agricultural soil; continuous accumulation is a concern to the produce safety and quality because of chronic human exposure. The inter-elemental correlation analysis showed high positive correlation between the cabbage and soil heavy metals which suggest common source of inputs. The soil-cabbage transfer factors of the heavy metals showed elevated level of Ni absorption and bio-accumulation into the dense leave head. The soil properties revealed poor nutrient status and this may inform the high usage of chemical fertilizer to improve yield, which pose threat to the cabbage quality and safety. Therefore, it is recommended that usage of organic manure to enhance soil fertility should be considered in order to limit accumulations of residual chemicals in the soil and crop.
ACKNOWLEDGEMENTS
- The authors are grateful to the University of Namibia, Katima Mulilo campus for funding this research. We also thank Mrs. Bertha Maswahu for her cooperation in the provision of samples for this study. Finally, we thank the management of Analytical Laboratory Services, Windhoek Namibia for facilitating the laboratory analysis of the study. God bless.
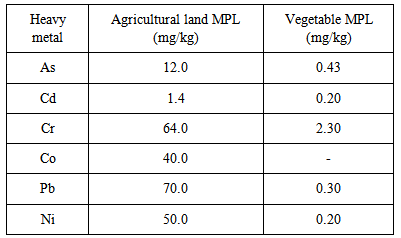
Appendix 1. Maximum Permissible Limits (MPL) of the Heavy Metals in Agricultural Land [28] and Vegetables [23]
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML