-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(3): 97-100
doi:10.5923/j.chemistry.20140403.02
N4O3 Potentially Heptadentate Schiff Base Ligand in the Synthesis of d/f Trimetallic Metal Complexes
Abdullahi Mustapha1, Suleiman Musa Gani2, Muhammad Saleh Salga2
1Department of Chemistry, Federal University Dutse, PMB 7156, Jigawa State
2Department of Pure and Industrial Chemistry, Umaru Musa Yar’adua University Katsina
Correspondence to: Abdullahi Mustapha, Department of Chemistry, Federal University Dutse, PMB 7156, Jigawa State.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Potentially heptadentate (N4O3) tripodal Schiff-base ligand: tris(5-Bromo-2-hydroxybenzylaminoethyl)amine (TrenSal) have been prepared and characterized by various spectroscopic methods. It is derived from the condensation reactions of tris(2-aminoethyl)amine (Tren), with 3 equivalents of 5-bromo-2-hydroxybenzaldehyde. Three new heteronuclear trimetallic complexes ware prepared by complexing monometallic (MnII), (FeII), and (CoII) with lanthanum (LaIII) metal ion to produce complexes the following complexes [LaCo2(Tren5BrSal)2]Cl, [LaMn2(Tren5BrSal)2]Cl and [LaFe2(Tren5BrSal)2]Cl.
Keywords: Trimetallic, Schiff base, Heptadentate, Lanthanum
Cite this paper: Abdullahi Mustapha, Suleiman Musa Gani, Muhammad Saleh Salga, N4O3 Potentially Heptadentate Schiff Base Ligand in the Synthesis of d/f Trimetallic Metal Complexes, American Journal of Chemistry, Vol. 4 No. 3, 2014, pp. 97-100. doi: 10.5923/j.chemistry.20140403.02.
1. Introduction
- Several studies showed that the presence of a lone pair of electrons in the Sp2 hybridized orbital of nitrogen atom of the azomethine group is of considerable chemical and biological importance [1, 2]. Because of the relative easiness of preparation, synthetic flexibility, and the special property of C=N group, Schiff bases are generally excellent chelating agents [2, 4, 5, 6] especially when a functional group like –OH or –SH is present close to the azomethine group so as to form a five or six membered ring with the metal ion. Versatility of Schiff base ligands and biological, analytical and industrial applications of their complexes make further investigations in this area highly desirable [7].Multidentate salicylidene Schiff base (N4O3) ligands have been chosen for study, due to the ligand ability to encapsulate metal cations in such a manner that the three terminal phenoxide donors assemble into a secondary binding motif to form six ring coordinating size. When divalent metals (e.g., Ni, Zn, Pb) are used, a charge imbalance between the encapsulated cation and the ligand is created, which leaves a residual charge on the complex. This facilitates the binding of an additional metal center between pairs of complexes [8]. Recently, the scope of this research was extended to the construction of oligometallic lanthanide complexes and mixed lanthanide/transition metal ion arrays. In this latter work the coaggregation of nickel (NiII) and lanthanide (LnIII) metal ions in the presence of a tripodal amine phenol ligand, H3-tam [1,1,1-tris (((2`-hydroxybenzyl) amino) methyl) ethane], provided a series of LnNi2 trinuclear complexes that were characterized in terms of their crystal structures and magnetic properties [9]. The synthesis and physical characterization of a series of lanthanide (LnIII) and nickel (NiII) mixed trimetallic complexes with the heptadentate (N4O3) ([tris(2`-hydroxybenzylaminoethyl)amine]) amine phenol ligand has been accomplished [10].Investigations concerning the structural and chemical properties of polynuclear transition-metal compounds are receiving increasing interest. The area has important implications for topics such as the nature of orbital interaction, electron transfer in redox reaction processes, and biological electron-transport chains. Trinuclear copper clusters have been found at the active sites of multicopper blue oxidases such as ascorbate oxidase, laccase and ceruloplasmin [11]. In this paper we report the synthesis of potentially heptadentate Schiff-base ligand derived from condensation of 5-bromo-2-hydroxy benzaldehyde with tris-(2-aminoethyl) amine and complex it with either Manganese (II) or Iron(II) or Cobalt (II) and Lanthanum(III) metal salt, in the ratio of 2:2:1.
2. Experimental
- All experiments were carried out using standard apparatus and the chemicals were of commercial quality and were used without further purification. The IR was measured on a Bruker FT-IR 8400s for the compound in the range 4500-500cm-1 and the UV-Vis was recorded in the range 280-750mm wavelength. The decomposition temperature was obtained using capillary tube and molar conductance of 10-3M was determined at room temperature. Elemental analysis and mass spectra were run at Al-azhar University Cairo Egypt.Preparation of Tren5BrSal Tris(2-aminoethyl)amine (1.46g, 10mmol) was added to a solution of 5-bromosalicylaldehyde (6.03g, 30mmol) in 100ml ethanol. The resulting solution was refluxed for 40mins. A yellow powdered solid was precipitated upon cooling for 24h. The product was filtered off, washed with ethanol, and allowed to air dried. Yield 77.7% [8].Synthesis of [Co2(Tren5BrSal)2La]ClTren5BrSal (1.0g, 1.4mmol) was dissolved in ethanol (50ml) followed by addition of CoCl2.6H2O (0.342g, 1.4mmol) in ethanol (10ml). The mixture was stirred after which few drops of triethylamine was added. LaCl3.7H2O (0.267g, 0.7mmol) in ethanol (10ml) was also added. The solution was refluxed 60 minutes. A shiny dark green solid was obtained from the filtrate when allowed to air dry. Yield 63%, [12].Synthesis of [Fe2(Tren5BrSal)2La]ClTren5BrSal (1.0g, 1.4mmol) was dissolved in ethanol (50ml) followed by addition of FeCl3.6H2O (0.389g, 1.4mmol) in ethanol (10ml). The mixture was stirred after which few drops of triethylamine were added followed by addition of LaCl3.7H2O (0.267g, 0.7mmol) in 10ml ethanol. The solution was refluxed for 60 minutes and was allowed to cool over night. The dark purple Solid was filtered off and allowed to air-dry. Yield 71%. Synthesis of [Mn2(TrenSal)2La]ClTrensal (1.0g, 1.4mmol) was dissolved in ethanol (50ml) followed by addition of MnCl2.4H2O (0.285g, 1.4mmol) in 10ml ethanol. The mixture was stirred after which few drops of triethylamine was added. LaCl3.7H2O (0.267g, 0.7mmol) in 10ml ethanol was also added. The solution was refluxed for 60 minutes. The resulting solution was allowed to cool over night. The grey-yellow Solid was filtered off and allowed to air-dry. Yield 52%.
3. Results and Discussion
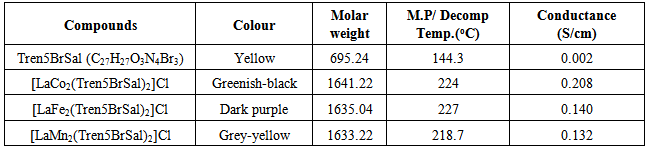
- The cobolt-lantanum complex was obtained as dark green solid, [LaFe2(Trensal)2]+ was obtained as dark purple solid while [LaMn2(TrenSal)2]+ was found to be of grey-yellow color. All the complexes showed certain level of stability with respect to heat as their decomposition temperature is above 200℃, see table 1.
|
|
 π*) and (n
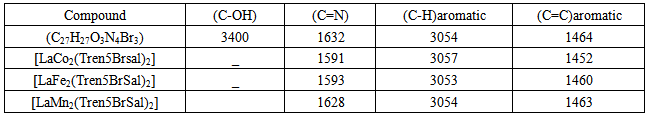
π*) and (n π) transitionThe intense greenish-black color of [Co2(TrenSal)2La]+ complex, showed a strong band at λmax 610nm and another band with low intensity at λmax 580nm which are attributed to charge transfer and electronic transition that took place as a result of complexation. The dark purple [Fe2(TrenSal)2La]+ complex shows an absorption band at λmax 530nm. However, the grey-yellow [Mn2(Tren5BrSal)2La]+ complex showed a strong absorption band at 540nm and another band at λmax 360nm which are also attributed to charge transfer and electronic transition in the complex formation. Infrared Spectral studiesThe characteristic vibrations and assignment of the Schiff base ligand and its complexes with La-Co, La-Fe and La-Mn are described in table 3.
π) transitionThe intense greenish-black color of [Co2(TrenSal)2La]+ complex, showed a strong band at λmax 610nm and another band with low intensity at λmax 580nm which are attributed to charge transfer and electronic transition that took place as a result of complexation. The dark purple [Fe2(TrenSal)2La]+ complex shows an absorption band at λmax 530nm. However, the grey-yellow [Mn2(Tren5BrSal)2La]+ complex showed a strong absorption band at 540nm and another band at λmax 360nm which are also attributed to charge transfer and electronic transition in the complex formation. Infrared Spectral studiesThe characteristic vibrations and assignment of the Schiff base ligand and its complexes with La-Co, La-Fe and La-Mn are described in table 3.
|
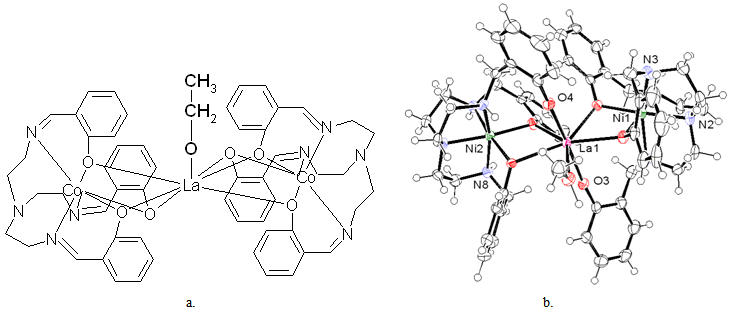 | Figure 1. The proposed mixed trimetallic structure of the [LaCo2(Tren5BrSal)2] [b. 10] |
4. Conclusions
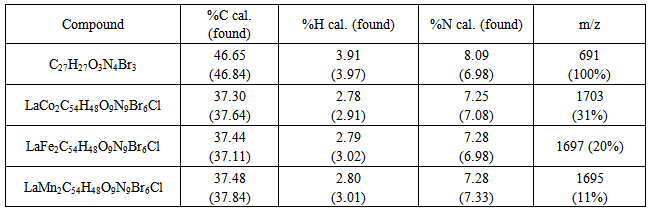
- We have successfully synthesized mixed trimetallic complexes using potentially (N4O3) heptadentate ligand. The complexes could not dominate the mass spectra (100%) due to its larger size, as such it is not the dominant peak. Further study on the complexes would be look into with regards to its magnetic property.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML