-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(2): 82-87
doi:10.5923/j.chemistry.20140402.03
Synthesis and Antimicrobial Screening of New Chalcones and 1,5-Benzothiazepines Containing Quinoline Nucleus
Kamal M. El-Gaml
Organic Chemistry Department, Al-Azhar University, Nasr City, Cairo, 11884, Egypt
Correspondence to: Kamal M. El-Gaml, Organic Chemistry Department, Al-Azhar University, Nasr City, Cairo, 11884, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
New series of benzo [b][1,5]-thiazepinederivatives 7a-hwere synthesized by applying the cyclocondensation of 1-(2-chloro-substituted quinolino-3-yl)-3-(4-substituted phenyl) prop-2-en-1-one derivatives 6a-h with o-aminothiophenol in DMF. The new intermediate Chalcone derivatives 6a-hwere obtained from interaction of benzaldehyde derivatives and 2-chloro-3-acetylquinoline derivatives (5). The synthesized 1,5-benzothiazepines 7a-hhave been screened for their antimicrobial activity.
Keywords: Chalcones, 1, 5-benzothiazepines,Antimicrobial activity
Cite this paper: Kamal M. El-Gaml, Synthesis and Antimicrobial Screening of New Chalcones and 1,5-Benzothiazepines Containing Quinoline Nucleus, American Journal of Chemistry, Vol. 4 No. 2, 2014, pp. 82-87. doi: 10.5923/j.chemistry.20140402.03.
Article Outline
1. Introduction
- Chalcones constitute an important class of natural products and some of them possess a wide range of pharmacological activities such as anticancer, anti-tubercular, antiviral [1]. Recent studies on biological evaluation of Chalcones revealed some to be antibacterial, antifungal, Anti-inflammatory, anti hyperglycaemic [2], and antimalarial agents [3]. 1, 5-Benzothiazepine nucleus has been well proved as illustrated by a large number of patents chemotherapeutic covering their utilities. A number of biological activities such as anticonvulsant [4], Ca+2 channel antagonist [5], antianginal [6], anti-HIV [7], squalene synthetase inhibitor [8], V2 arginine vasopressin receptor antagonist [9], etc. As a continuation of our interest in polycyclic, we investigated the combination of the thiazepine and quinoline in thecommon polyheterocyclic system, because synthesis of such compounds show diverse pharmacological activities. Here in the present work an attempt has been made to synthesize new Chalcones and 1,5-benzothiazepines incorporating heteryl pharmacophores such as quinoline in a single molecular frame work, with the hope to obtained new molecules with enhanced biological activity. Literature reveals that the present work deals with the reaction of 6-substituted -2-chloro-3-acetylquinoline with different aromatic aldehydes to form Chalcones 6a-h, and the success of the entire various synthesized compound were assigned on for basis of elemental analysis, and spectral data.
2. Experimental
2.1. General
- Melting points were measured in capillary tube on a Graffin melting point apparatus and are uncorrected. The IR spectra were recorded on PyeUnicam SP 1000 IR spectrophotometer using KBr discs (λmax in cm-1). 1HNMR spectra were performed either on a Jeol ECA (500 MHz) or Gemini 300BB (300MHz) spectrometer, using TMS as internal standard and DMSO-d6 as solvent; the chemical shifts are reported in ppm (δ) and coupling constant (J) values are given in Hertz (Hz). Signal multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quadruplet), and m (multiplet). All of the new compounds were analyzed for C, H and N and agreed with the proposed structures within ±0.4% of the theoretical values by the automated CHN analyzer at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Mass spectra were recorded on Hewlett Packard 5988 spectrometer at the RCMB. The purity of the compounds was checked by thin layer chromatography (TLC) on Merck silica gel 60 F254 precoated sheets. The starting Compounds 1-5 were prepared according to reported procedures [10-14].
2.1.1. Chemistry
- The synthesis of the target compounds was achieved according to steps in Scheme 1. Hydrolysis of 1with H2SO4 (70%) in ethanol produce substituted 2-oxo1,2-dihydroquinoline-3-carboxylic acids (2), which when reacted withphosphoryl chloride it gave substituted 2-chloroquinoline-3-carbonyl chloride (3), that in the presence of N,O-dimethyl hydroxylamine and THF give 2-chloro-N-methoxy-N-methylquinoline-3-carboxamide derivatives (4a-d) that dried on further reaction with methyl magnesium bromide in ammonium chloride and etherat room temperature gave 2-chloro-3-acetylquinoline derivatives (5a-d), the structure of compounds 5 a-d was confirmed on the basis of spectral data.
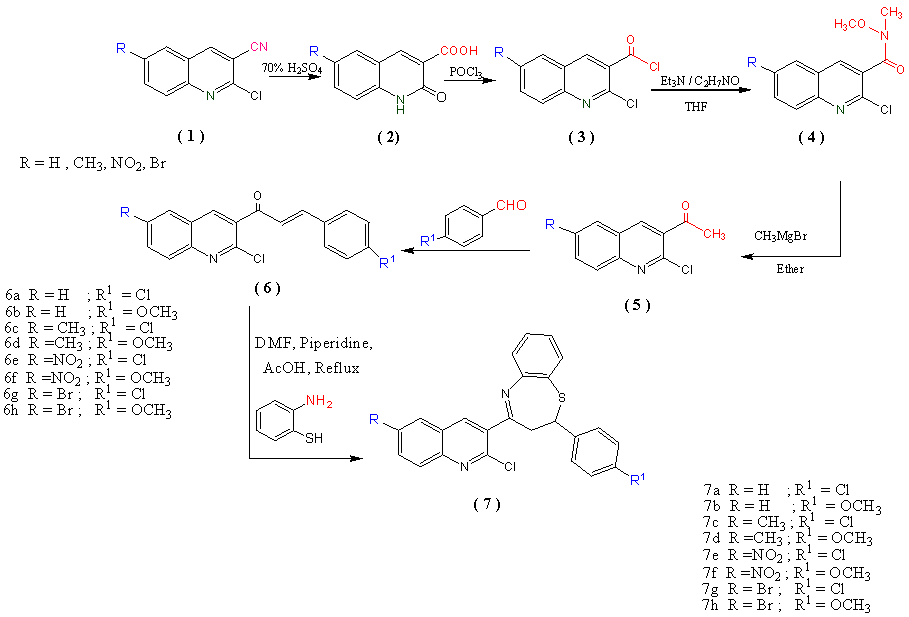 | Scheme 1 |
2.1.2. General Procedure for the Synthesis of 1-(2-Chloro-6-substituted-quinoline-3-yl)-3-(4-substituted phenyl) prop-2-en-1-ones (6a-h)
- A mixture of substituted-2-chloro-3-acetylquinoline (0.002 mol) and potassium hydroxide (0.0058 mole) was dissolved in ethanol (15mL). To this solution 4-substituted- benzaldehyde (0.002 mol) in was added in portion at 0-5℃ with constant stirring. To this reaction mass catalytically amount of phase transfer catalyst, tetrabutyl ammonium bromide was added and then reaction mixture was further stirred for 3 h, by keeping the reaction mixture in ice. It was then allowed to stand at room temperature for overnight. The reaction progress was monitored by TLC. Then the reaction mass was poured on crushed ice and neutralized by glacial acetic acid. The solid appeared was filtered, washed with cold water and crystallized from ethanol: ethyl acetate mixture, the structure of the resulting compound was confirmed on the basis of spectral data Table 1.
|
2.1.3. General procedure for the Synthesis of 4-(2-Chloro-6-substituted quinoline-3-yl)-2-(4-substituted phenyl) 2,3-dihydro-benzo[b][1,5] thiazepine(7a-h)
- A mixture of (6) and 2-aminothiophenol (0.0022 mol) was dissolved in DMF (10mL). A few drops of piperidine were added to the solution and it was then refluxed for 4 h. It was then acidified with glacial acetic acid (2 mL) and reaction mixture was further refluxed for 2 hrs. The progress of the reaction was monitored by TLC. After completion the reaction mixture was left overnight at room temperature. Then the reaction mass was poured in ice cold water and the obtained solid was filtered. The crude was crystallized form EtOH: DMF. the structure of compounds 7 a-h were confirmed on the basis of analytical and spectral data .2-(4-chlorophenyl)-4-(2-chloroquinolin-3-yl)-2,3-dihydrobenzo[b][1,5]-thiazepine (7a)Yield 70 %, mp 126-127℃. MS m/z: 435(8.4, M+), 437 (3.1, M+2). Anal.Calc. For C24H16 Cl2N2S: C, 66.21; H, 3.70; N, 6.43. Found.C, 66.44; H, 3.61; N, 6.62.4-(2-chloroquinolin-3-yl)-2-(4-methoxyphenyl)-2,3-dihydrobenzo[b][1,5]-thiazepine (7b)yield 74%, mp 129-130°C. IR (KBr) cm-1: 3060, 2910, 1620. 1HNMR(DMSO-d6) δ: 2.34 (s, 3H, -OCH3), 3.01-3.04 (dd, 1H, CH2-HA, JAX = 5.80Hz, JAB = 12.50 Hz), 3.05-3.08 (dd, 1H, CH2-HB, JBX = 7.77 Hz, JBA = 12.50Hz), 3.15 (s, 3H,-OCH3), 3.11-3.14 (t, 1H, CH-Hx, JXA = 2.50Hz, JXB = 7.50Hz), 6.20-6.23(t,2H, H4, H5- phenyl ), 6.30-6.33(d, 2H, H3, H6 -phenyl),6.40-6.42(d, 2H, H3, H5-phenyl-OCH3), 6.32-6.34(d, 2H, H2, H6-phenyl-OCH3),7.42-7.44(t, 1H, H6-quinoline), 7.28-7.52(s, 1H, H5-quinoline),7.58-7.60(d , 1H , H7,-quinoline), 7.64-7.66(d, 1H,H8, -quinoline),8.10(s, 1H, H4, quinoline). MS m/z:430.95 (24, M+), 432.12 (7.8, M+2). Anal.Calc. For C25H19 ClN2OS: C, 60.68; H, 4.44; N, 6.50.Found.C, 69.73; H, 4.45; N, 6.80.4-(2-chloro-6-methylquinolin-3-yl)-2-(4-chlorophenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7c)yield 80%, mp 123-124°C. MS m/z: 449.39 (31.2, M+), 451 (9.9, M+2). Anal.Calc. For C25H18 Cl2N2S: C, 66.82; H, 4.04; N, 6.23.Found.C, 69.56; H, 4.23; N, 6.36.4-(2-chloro-6-methylquinolin-3-yl)-2-(4-methoxyphenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7d)yield 95%, mp 136-137°C, IR (KBr) cm-1: 3059, 2924, 1621. 1H NMR (DMSO-d6) δ: 2.37 (s, 3H, CH3), 3.55(s, 3H-OCH3), 3.00-2.03(dd, 1H, CH2-HA, JAX = 5.94Hz, JAB = 13.50 Hz), 3.04-3.07 (dd, 1H, CH2-HB, JBX = 7.73 Hz, JBA = 13.50Hz),),2.65-2.68(t,1H, H2, CH-Hx, JXA = 2.54Hz, JXB = 7.82Hz), 6.19-6.21(t, 2H, H4, H5-phenyl), 6.30-6.32(d, 2H, H3, H6 -phenyl), 6.38-6.0(d, 2H, H3, H5-phenyl-OCH3), 6.31-6.33(d, 2H, H2, H6-phenyl-OCH3),7.25-7.27(s, 1H, H5-quinoline),7.42-7.45(d,1H , H7,-quinoline), 7.67-7.69(d, 1H, H8, -quinoline ),8.02(s, 1H, H4, quinoline ). MS m/z: 444 (33, M+), 446 (11.2, M+2). Anal.Calc. For C26H21 ClN2OS: C, 70.18; H, 4.76; N, 6.30.Found.C, 70.22; H, 4.68; N, 6.25.4-(2-chloro-6-nitroquinolin-3-yl)-2-(4-chlorophenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7e)yield 55%, mp 155-156°C. IR (KBr) cm-1: 3070, 2995, 1614. 1HNMR (DMSO-d6) δ:2.02-2.05(dd, 1H, CH2-HA, JAX = 6.02Hz, JAB = 15.10 Hz), 3.04-3.07 (dd, 1H, CH2-HB, JBX = 8.01 Hz, JBA = 15.10Hz),2.62-2.65(t,1H, H2, CH-Hx, JXA = 2.63Hz, JXB = 7.87Hz),6.19-6.21(d, 2H, H4, H5-phenyl), 6.30-6.32(d, 2H, H3, H6-phenyl), 6.70-6.72(d, 2H, H2, H6-phenyl-Cl), 6.92-6.94(d, 2H, H3, H5-phenyl-Cl), 8.05-8.07(s, 1H, H4-quinoline),8.12-8.14(d,1H, H8, -quinoline), 8.17-8.19(d, 1H, H7, -quinoline ),8.22(s, 1H, H5, quinoline). MS m/z: 480.37 (17, M+). 482 (6.1, M+2). Anal.Calc. For C24H15 Cl2N3O2S: C, 60.01; H, 3.15; N, 8.75.Found.C, 60.33; H, 3.42; N, 8.42.4-(2-chloro-6-nitroquinolin-3-yl)-2-(4-methoxyphenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7f)yield 60%, mp 155-156°C. MS m/z: 475.95 (24, M+), 477 (8.2, M+2). Anal.Calc. For C25H18 ClN3O3S: C, 63.09; H, 3.81; N, 8.83.Found.C, 63.15; H, 3.71; N, 8.72.4-(6-bromo-2-chloroquinolin-3-yl)-2-(4-chlorophenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7g)yield 65%, mp 150-151°C. MS m/z: 514.26(29.2, M+), 516 (9.7, M+2). Anal.Calc. For C24H15 BrCl2N2S: C, 56.05; H, 2.94; N, 5.45.Found.C, 56.18; H, 3.12; N, 5.11.4-(6-bromo-2-chloroquinolin-3-yl)-2-(4-methoxyphenyl)-2,3-dihydrobenzo[b]-[1,5]thiazepine (7h)yield 70%, mp 142-143°C, IR (KBr) cm-1: 3075, 2998, 1612 .1HNMR(DMSO-d6) δ: 1H NMR (DMSO-d6) δ: 3.53(s, 3H-OCH3), 2.11-2.14(d, dd, 1H, CH2-HA, JAX = 6.02Hz, JAB = 12.02 Hz),), 2.12-3.15 (dd, 1H, CH2-HB, JBX = 6.01 Hz, JBA = 12.02Hz),2.65-2.67(t, t,1H, H2, CH-Hx, JXA = 2.44Hz, JXB = 7.32Hz), 6.17-6.19(d, 2H, H4, H5 -phenyl), 6.33-6.34(d, 2H, H3, H6 -phenyl), 6.43-6.45(d,2H, H3, H5 -phenyl-OCH3), 6.63-6.65(d, 2H, H2, H6 -phenyl-OCH3), 8.02(s,1H, H4 -quinoline), 8.15-8.17(d,1H, H8 -quinoline),8.22-8.25(d,1H, H7-quinoline), 8.67-7.69(s,1H, H5 -quinoline), MS m/z: 480 (19.7, M+). 482 (6.9, M+2). Anal.Calc. For C25H18 BrClN2OS: C, 58.89; H, 3.56; N, 5.49.Found.C, 58.61; H, 3.78; N, 5.41.
3. Result and Discussion
- The keyprecursor,2-oxo-1,2-dihydroquinoline-3-carboxylic acid derivatives which prepared according to the reported procedure [10-14]. The structures of compound 2 were confirmed by 1HNMR,13C-NMR, In the 1H-NMR spectra, where two sharp proton signal observed at the δ 5.4 and 11.68 ppm, one belong OH at C-2 and one belong COOH respectively, and due to resonance isomerism the NH (secondary amine) itappear at higher chemical shift at 8.9 as abroad signal more than expected due to neighbouring carbonyl group, In the 13C-NMR spectra of these compounds one C=O peak at about 178 ppm and one around 166 ppm corresponded to the signals of the 3-carboxylic acid and carbonyl groups in the quinoline rings. On contrary the IR spectra of compound 3 showed the disappearance of OH stretching band and this explained the conversion of acid into acid chloride and the mass spectra of compound 3 confirm the conversion of carbonyl at C-2 into chloride moiety, so when the compound 3react with N,O-dimethyl hydroxylamine it produce the compound 4 which IR spectra confirm the presence of these compound because it showed clearly decreasing of stretching absorption band of C=O from 1812 cm-1 and appearance of sharp medium stretching absorption bands at range of 1636 cm-1 which belong to (C=O) for amide, when compound 4 react with alkyl Grignard reagent it produce compound 5, in which carbonyl appear at 1680-1695cm-1 as reported [14] hence when 5 react with benzaldehyde derivatives it afford new Chalcones 6, the IR spectra of new Chalcones have a two stretching bands at 1660-1590, this due to C=O and CH=CH, in addition the 1HNMR of Chalcone have two doublet signal one at 7.40 ppm which belong CHβ, and one at 6.90 which belong CHα. upon cyclocondensation of Chalcones with o-aminothiophenol it give new1,5-benzothiazepine derivatives 7, where the elemental analysis and spectral data confirm the existence of this cyclocondensation in which the 1HNMR of new 1,5-benzothiazepines contain two signal at 2.72 and 3.99 ppm that prove the cyclization well occur and presence of one hydrogen at C-2 and two hydrogen at C-3 of thiazepine ring . In addition the disappearance of sharp stretching of carbonyl and appearance of starching at 1590 (C=N of thiazepine) and all this fact will prove this cyclocondensation reaction and formation of 1, 5-benzothiazepine ring.Antimicrobial activity: The cup plate agar diffusion method [15] was employed for determining the antimicrobial activity of the newly synthesized compounds 6, 7 against two gram positive bacteria viz., Bacillus subtilis, Staphylococci aureus and two gram negative bacteria viz., Escherichia coli, Pseudomonas aeruginosa in addition to fungi (Candida albicans). The solutions of different compounds under test at a concentration of 500 and 600 μg/ml in 5% DMSO were poured in the cup/well of bacteria seeded agar plates. These plates were incubated at 37oC for 24 hours for E. coli, whereas plates of other three bacteria were incubated at 27oC for 24 hr. The standard antibiotics used were ampicillin (all at 500 μg/ml). and standard antifungal used were nystatinat 500 μg/ml, The control solution (only 5% DMSO) did not reveals any inhibition. The zone of inhibition produced by each compound was measured in mm. The results of antimicrobial studies are given in Table 1. The discussion and comparison of antibacterial activity were given with respect to ampicillin antibiotic and antifungal screening was compared with Nystatin.
4. Conclusions
- We have synthesized a series of new Chalcones6a-h and 1, 5-benzothiazepines 7a-h containing bioactive heteryl pharmacophores such as quinoline using convenient method. The antimicrobial activity of representative Chalcones 6a-h showed veryweak degree of, but the testing 1, 5-benzothiazepines VIIa-h showed moderate degree of antimicrobial activity. Microbiological testing of the newly synthesized compounds was performing in the Regional Center for Mycology and Biotechnology, Department of Microbiology, Faculty of Science, Al-Azher University, Cairo, Egypt.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML