-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(2): 78-81
doi:10.5923/j.chemistry.20140402.02
Reactions of Inorganic Tin (IV) and Lead (II) Compounds with Mono- and Bi-dentate Ligands Having Nitrogen and Oxygen Donors
Burl Yearwood
Department of Natural Sciences, LaGuardia Community College (CUNY), Long Island City, NY, USA
Correspondence to: Burl Yearwood, Department of Natural Sciences, LaGuardia Community College (CUNY), Long Island City, NY, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
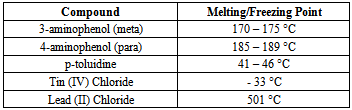
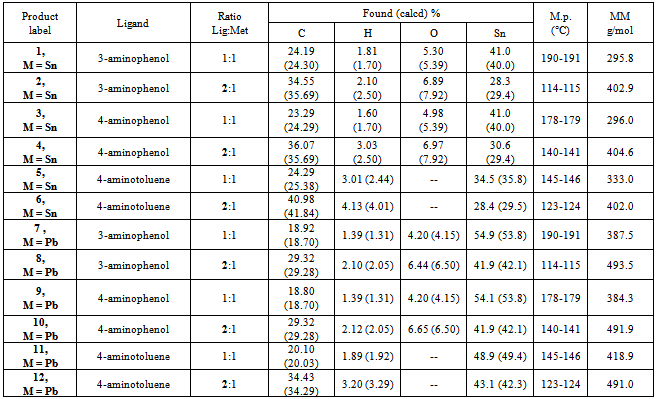
Tin (IV) chloride pentahydrate and Lead (II) chloride were reacted with meta and para aminophenol, and p-toluidine (4-aminotoluene), to determine if the reaction occurs via an elimination reaction or by adduct formation. The products of the metal-ligand reactions were characterized by elemental analysis, melting point, molecular mass determinations, and FT-IR spectroscopy. Melting points tests, at various times after the reaction, showed that the products were relatively air-stable. Infra-red spectroscopy revealed that the metal bonded to oxygen and/or nitrogen of the ligand. The FT-IR spectra showed the disappearance of absorption bands corresponding to the phenolic OH group and/or aromatic amino group in the starting ligands. Molecular mass determinations show the formation of a monomer unit when a 1:1 mole ratio of ligand to metal is used. A dimer unit is formed when a 2:1 mole ratio of ligand to metal is reacted. These results demonstrate that Tin (IV) chloride pentahydrate and Lead (II) chloride react with the mono and bi-dentate ligands used in this study to produce complexes via an elimination reaction, rather than adduct formation.
Keywords: Tin (IV) chloride, Lead (II) chloride, Aminophenol, 4-aminotoluene
Cite this paper: Burl Yearwood, Reactions of Inorganic Tin (IV) and Lead (II) Compounds with Mono- and Bi-dentate Ligands Having Nitrogen and Oxygen Donors, American Journal of Chemistry, Vol. 4 No. 2, 2014, pp. 78-81. doi: 10.5923/j.chemistry.20140402.02.
1. Introduction
- Our research is based on the reactions of organic and inorganic Tin, Lead, and Mercury compounds with multi-dentate ligands. The removal of Tin, Lead, and Mercury compounds from the environment is considered a top priority for local, state, and federal governments as these metals are very toxic to biological life [1-5]. In this study, we investigate whether inorganic Tin and Lead Chlorides will bind with certain mono- and bidentate ligands. These reactions will serve as a starting point to investigate the possible use of these mono- and bidentate ligands as environmental remediation agents. Our research focuses on the preparation and use of air stable compounds. Most organotin and organolead compounds prepared using similar reagents tend to be air-sensitive [6]. The ligands used in this study are meta and para aminophenol (3-aminophenol and 4-aminophenol respectively), and p-toluidine (4-aminotoluene). The effect of differing mole ratios of ligand to metal was also investigated. It is proposed that the ligands used in this study bind to the metal via an elimination reaction rather than by adduct formation (Figures 1 and 2).
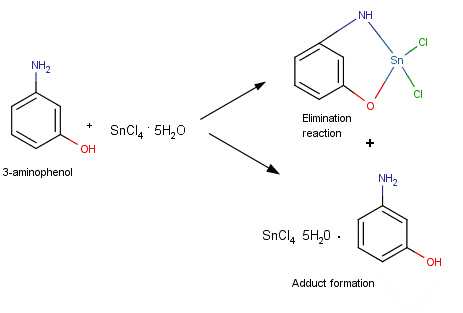 | Figure 1. Schematic showing proposed reaction schemes between 3-aminophenol and Tin (IV) Chloride pentahydrate in a 1:1 mole ratio |
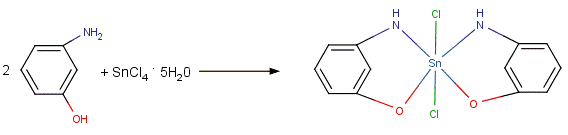 | Figure 2. Schematic showing proposed reaction schemes between 3-aminophenol and Tin (IV) Chloride pentahydrate in a 2:1 mole ratio |
2. Procedure
- For all experiments the same basic procedure was used. A specific example is shown below. For each mono- and bidentate ligand, experiments were carried out with a 1:1 mole ratio, and a 2:1 mole ratio between ligand and metal respectively. Each mole ratio reaction was carried out at room temperature. Reaction of meta-aminophenol with Tin (IV) Chloride pentahydrate (2:1 mole ratio).Meta-aminophenol (0.939 g, 0.0086 mol) was stirred into a mixture of isopropanol (8.0 mL) and ethanol (7.0 mL). To this, triethylamine (1.20 mL) was added. The resulting solution was stirred. In a separate beaker a solution of Tin (IV) Chloride pentahydrate (1.5 g, 0.0043mol) was dissolved in isopropyl alcohol (20.0 mL) and ethanol (10.0 mL). The solution containing the phenol was added to the solution containing the tin chloride. A white solution was formed. The resulting solution was stirred at room temperature for 24 hours. The white solution turned yellow. The solvent was removed to leave behind a yellow solid. A melting point determination, elemental analysis, and a FT-IR were carried out on the solid. The molar mass of the product was determined by using the Rast method [7]. Solubility tests were also performed on the yellow solid.
3. Results and Discussion
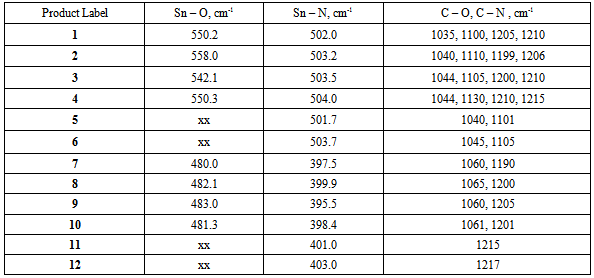
- The IR spectra of the starting ligands and the products of their reactions with Tin (IV) chloride pentahydrate and Lead (II) chloride were recorded. Some important assignments are show in Table 3. A significant feature in the spectra of the products is the absence of broad v(OH) bands for the phenolic OH groups expected in the region 3000 - 3700 cm-1, and the absence of the v(NH2) bands for the aromatic amino groups expected in the region 3300-3400 cm-1. This indicates oxygen and/or nitrogen bonding to the Tin moiety after replacement of the phenolic and/or amino hydrogen. In adduct formation; the phenolic OH group and/or the aromatic NH2 group would still be present in the final product. Further evidence for bonding between the metals and the ligands is seen in the IR frequencies for the new Sn-O and Pb-O bonds. The Sn-O frequencies are observed in the range of 550-560 cm-1 for compounds 1-4. The IR spectra also show that the metals also form a bond to the other binding site of the aminophenols, with the formation of Sn-N and Pb-N bonds. The Sn-N frequencies are observed in the range of 501-504 cm-1 for compounds 1-6. The v(Sn-O) modes are reported to be at 613–515 cm-1 for different organotin(IV) complexes of Schiff bases and at 500 ± 10 cm-1 for some methyltin(IV) complexes [8-11]. Frequencies for v(Sn-N) have been reported at 450-500 cm-1 for organotin salicylideneimine ligands [8-13].The Pb-O frequencies are observed in the range of 480-483 cm-1 for compounds 7-10. As with the tin complexes, the lead compounds also bind to the nitrogen of the ligands. The Pb-N frequencies are observed in the range of 395-403 cm-1 for compounds 7-12. The v(Pb-O) modes have been reported for organolead salicylideneimine ligands at 450-531cm-1, and v(Pb-N) at 305-350cm-1 [14].The IR spectra thus suggest that the metal coordinates to the ligand in the fashion shown in Figures 1 and 2.Melting point determinations confirmed that the products of the reactions were different from the starting materials, demonstrating that a chemical reaction had taken place between the metal halides and ligands. After the solid reaction products had been purified, melting points were carried out on the stored solids several days apart. The melting points were similar over a period of 1-2 weeks (data not shown), indicating the products were air stable.Molecular weight studies were carried out on the 1:1 and 2:1 molar ratio products to determine if the products were the monomer or dimer units. The Rast method for determining molecular weights was used, using camphor as the solvent. Table 2 shows that 1:1 mole ratio reactions produced monomer units, while 1:2 mole ratio (ligand:metal) produced dimer units (that is two ligands are bonded to the metal).The values obtained from the elemental analysis of the products are in good agreement with the proposed formulae, and further serve to support the idea of complexation of the metal to the ligand (as opposed to adduct formation), plus the idea of dimer formation when a 2:1 mole ratio of ligand to metal is used.
|
|
4. Conclusions
- Our studies show that inorganic Tin (IV) and Lead (II) Chlorides bind to the mono- and bi-dentate ligands used in this study by an elimination reaction pathway rather than by adduct formation. The presence of Sn-O and Sn-N bands in the IR, and the absence of OH and NH bands support this hypothesis. It has also been shown that varying the ligand to metal ratio has an effect on the structure of the product formed.
ACKNOWLEDGEMENTS
- To the Department of Natural Sciences and LaGuardia Community College for providing research facilities.
References
| [1] | P. Olmedo, A. Pla, A. F. Hernández, F. Barbier, L. Ayouni, F. Gil, 2013, Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environment international, 59, 63-72. |
| [2] | E. Dopp, S. Bhattacharya, A. V. Hirner, M. Aschner, T. Schwerdtle, 2012, Toxicity of Organometal (loids). Journal of toxicology. |
| [3] | A. Agrawal, 2012, Toxicity and fate of heavy metals with particular reference to developing foetus, Adv Life Sci, 2, 29-38. |
| [4] | S. Chatterjee, 2012, India’s Readiness on ROHS Directives: A Strategic Analysis, Global Journal of Science Frontier Research, 12,(1-E). |
| [5] | W. P. Ridley, L. J. Dizikes, and J. M. Wood, 1977, Biomethylation of toxic elements in the environment, Science, 197, 4301, 329-332. |
| [6] | Heavy Metals in the Environment: Origin, Interaction and Remediation,Volume 6, Bradl, Heike (editor), Academic Press, 2005. |
| [7] | Ullmann Fine Chemicals, 2014, Volume 1-3, Wiley-VCH (editor), John Wiley and Sons. |
| [8] | R. L. Shriner, R. C. Fuson, 1948, The Systematic Identification of Organic Compounds, 3rd ed., John Wiley and Sons Inc., New York, pp. 50-1. |
| [9] | B. Yearwood, S. Parkin, D. A. Atwood, 2002, Inorganica Chimica Acta, 333, 124. |
| [10] | H. I. Beltrán, C. Damian-Zea, S. Hernández-Ortega, A. Nieto-Camacho, 2007, Synthesis and characterization of di-phenyl-tin(IV)-salicyliden-ortho-aminophenols: Analysis of in vitro antitumor/antioxidant activities and molecular structures, Journal of inorganic biochemistry, 101(7), 1070-1085. |
| [11] | H. K. Fun, 1997, Polyhedron, 16, 2213. |
| [12] | A. K. Singh, S. Bhandari, 2003, Tin (II and IV) complexes with nitrogen ligands: Review of recent developments, Main group metal chemistry, 26.3, 155-211. |
| [13] | M.C. Kutcha, J. M. Hahn, G. Parkin, 1990, Divalent tin and lead complexes of a bulky salen ligand: the syntheses and structures of [SalenBut, Me] Sn and [SalenBut, Me] Pb, Journal of the Chemical Society, Dalton Transactions, 20, 3559-3563. |
| [14] | E. N. Sakuntala, E. N. Vasanta. 1985, Tin (IV) and organotin (IV) coordination complexes with nitrogen and oxygen (sulfur) containing ligands, Zeitschrift für Naturforschung. Teil b, Anorganische Chemie, organische Chemie, 40b, 1173-1176. |
| [15] | S. A. Sadeek, M. S. Refat, and S. M. Teleb, 2004, Spectroscopic studies and thermal analysis of lead (II) and tin (II) solid complexes with bi-, tri-and tetradentate Schiff bases, Bulletin of the Chemical Society of Ethiopia, 18.2. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML