-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(2): 73-77
doi:10.5923/j.chemistry.20140402.01
Electrical Conductance of α-VOPO4 2.5H2O
Mohamed M. K. Ellafi, Sadek K. Shakshooki
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: Sadek K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Layered α-vanadyl phosphate, α-VOPO4 2.5H2O, was prepared, found to be nanosized with average diameter equal to ~57.96 nm, which was calculated from the full width at half maximum of the peak, from XRD analysis, using Scherrer,s equation The layers are hold together by van der Waals bonds. It was characterized by XRD, TGA, FT-IR and scanning electron microscopy(SEM). The conductivity determination (σ) was made by ac-method at temperatures range (25-95C) on compact discs of the material using graphite electrodes. The conductivities (σ) were found to be 1.74x10-4Ω-1 cm-1, 2.51x 10-4 Ω-1 cm-1 and 7.17x10-4 Ω-1 cm-1. at 25, 60 and 95C, respectively. The conductivity (σ) is increased by increasing of temperature. The relation between the conductivity (σ) and the inverse of absolute temperature was linear in the two regions, the low temperature region (25 - 60℃) and the high temperature region (65 - 95℃). The activation energy was calculated for the two regions using the Arrhenius equation, and found to be 0.113 and 0.339 eV, respectively.
Keywords: Electrical conductance, α -VOPO4 2.5H2O, Activation energy
Cite this paper: Mohamed M. K. Ellafi, Sadek K. Shakshooki, Electrical Conductance of α-VOPO4 2.5H2O, American Journal of Chemistry, Vol. 4 No. 2, 2014, pp. 73-77. doi: 10.5923/j.chemistry.20140402.01.
Article Outline
1. Introduction
- Vanadium forms a variety of oxides with interesting potential applications. Yielding various compounds among them vanadyl phosphates, being widely used as commercial catalyst [1, 2]. Vanadyl phosphate can exist in several crystalline phases [3, 4] in both anhydrous and hydrated form. In layered hydrated vanadyl phosphate of general formula VOPO4.nH2O (where n = 2 - 2.5), the water molecule play an important role in electrical properties. The layers consist of distorted VO6 octahedra sharing all their equatorial oxygen's with four PO4 tetrahedra, the weakly bonded axial oxygen being replaced by a water molecule (coordinated water). The rest of water molecules are inserted into the space between PO4 tetrahedral of adjacent layers. All water molecules are linked together by hydrogen bonds making a double layer between VOPO4 sheets [5, 6]. The strong acidic character of vanadyl phosphate layers lead to formation of H3O+ ions in the interlayer region [6]. The layers are hold together by van der Waals bonds [3, 7].Proton conductors are often considered to be electrolytes in which hydrogen is transport forwards and evolved at the cathode during electrolysis. Proton transport include transport of proton (H+) and any assembly that carries protons (H+), H2O, H3O+, NH4+….etc. [5, 6]. The transport protons (H+) between relatively stationary host anions is termed "Grotthuss" or " free proton" mechanism and classical mass diffusion (vehicle mechanism). In Grotthus mechanism, hydronium ions are oriented in proper position for proton conduction. In vehicle mechanism proton transports by water molecule charge carrier as H3O+ [8 - 12]. Electrical current arises from migration of positive ions (H+) which are attached to negative [V-O]- on the layer. Strong electrostatic act between H+ and [V-O]- ions. At room temperature VOPO4.nH2O mixed protonic-electronic conduction with dominant protonic [5, 6]. In this paper we have investigated the electrical conductance of α -VOPO4 2.5H2O at different temperature ranges.
2. Experimental Details
2.1. Chemicals
- V2O5, H3PO4 (85%) (of BDH) were used as such. Other reagents used were of analytical grade.
2.2. Instruments
- X-ray powder diffract meter Philips, using Ni-filtered CuKα (λ = 1.54056Å), TG/DTA SII Extra 6000 thermogram. and TG/DTA Perkin-Elmer SII, Scanning electron microscopy (SEM) Jeol SMJ Sm 5610 LV, Forier Transform IR spectrometer, model IFS 25 FTIR, Bruker and pH Meter WGW 521.
2.3. Preparation of α -Vanadyl Phosphate
- α-Vanadyl phosphates, VOPO4.2.5H2O (VOP), was prepared as described previously [13]. In detail, 35 ml H3PO4 (85%) in 250 ml distilled water were added to 10g V2O5 with stirring at room temperature. The resulting mixture was stirred under reflux for 24 h. The resultant product, green olive precipitate, was filtered washed with cold ethanol and dried in air.
2.4. Conductivity Determination
- The conductivity determinations (σ) of layered α-vanadyl phosphate, α-VOPO4 2.5H2O, was measured by the usual ac-method and the sample holder was exactly the same as used in earlier conductivity and thermo-emf work [11, 15 -18] in form of compact discs of 1.2 cm in diameter was prepared at about 8x105 kP/cm2 pressure, area is 1.13cm2 and thickness of about 0.115 cm, at a temperature range 25 to 95℃ using graphite as electrodes. The material was heated at 80℃ for about one hour in dry atmosphere, left in air at room temperature (∼ 20℃) for 48 h till constant weight prior ac measurements.
3. Results and Discussion
- Layered α-vanadyl phosphate, was prepared and characterized by X-ray diffraction (XRD), Fourier Transform IR (FT-IR), thermo gravimetric analysis (TGA) and scanning electron microscopy (SEM).
3.1. XRD
- The X-ray diffraction pattern of crystalline layered α- VOPO4 2.5H2O is shown in figure (1), which indicates good degree of crystalline with interlayer spacing (d001) equal to 6.86Ǻ. The average diameter of α-vanadyl phosphate found to be equal to 57.96 nm which were calculated from the full width at half maximum of the peak, from XRD analysis, Figures (1,2), using Scherer's equation:
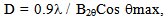 | (1) |
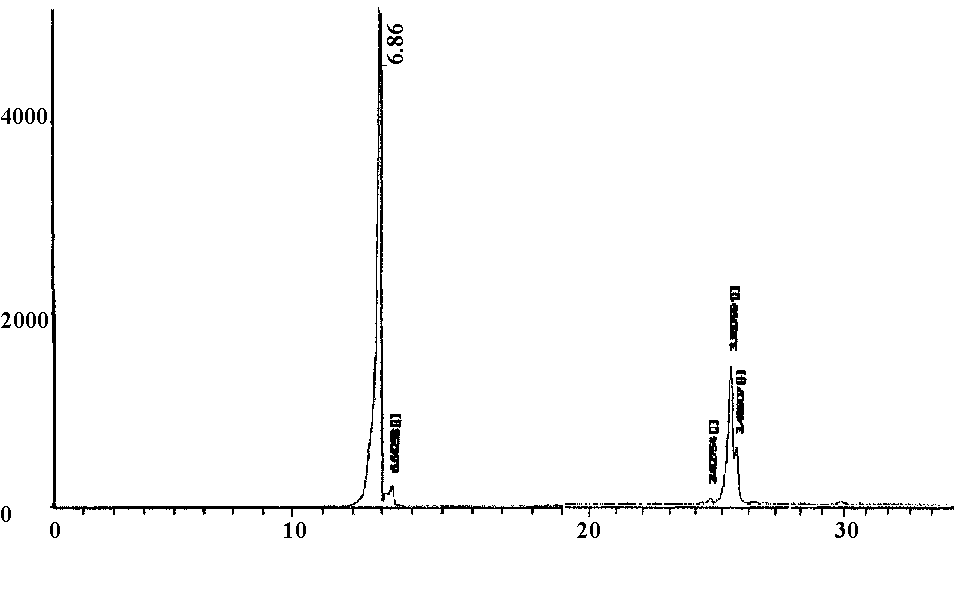 | Figure 1. XRD pattern of α- VOPO4.2.5H2O |
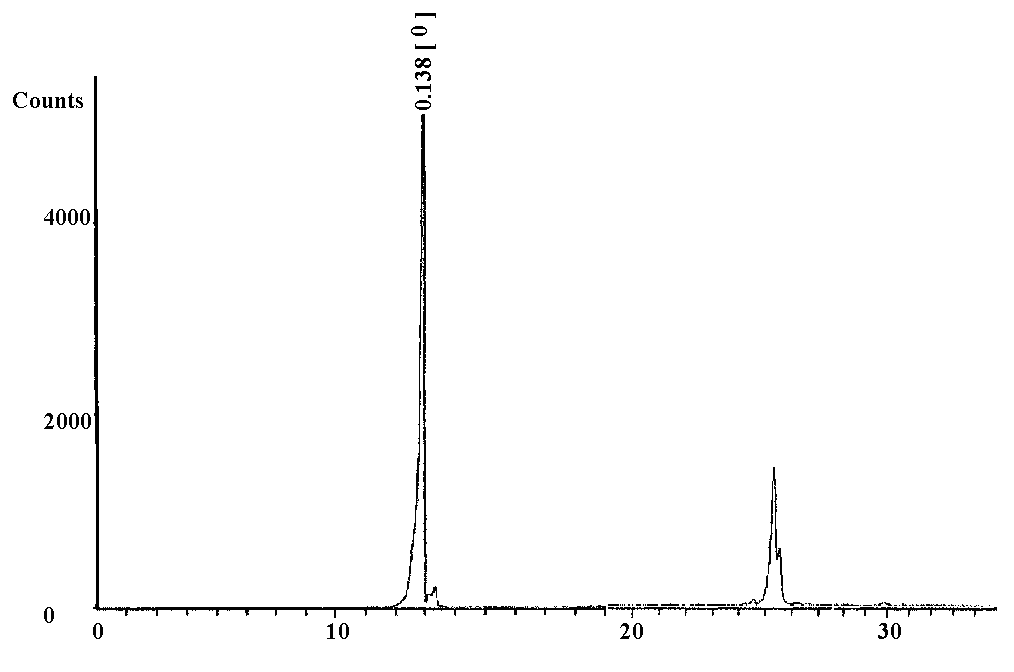 | Figure 2. XRD pattern of α -Vanadyl phosphate full width at half maximum of the peak which is 0.138 |
3.2. SEM
- Figure (3) show the scanning electron micrographs(SEM) of α-vanadyl phosphate VOPO4 2.5H2O. The image of the matrix demonstrates that the crystallites of α-vanadyl phosphate consists of irregular stacking of square plates. From the image of SEM we calculate the thickness of a square plate by carefully random selecting of three stacking square plates from the figure, as follows the figure, as follows (10 μm ≡ 7 mm. So 0.07mm thickness → 10μm x 0.07 mm/ 7mmx1000 = 100nm, 0.05mm=71.418nm, and 0.04mm = 57.142nm).
 | Figure 3. SEM miograph image of α –vanadyl phosphate |
3.3. FT-IR
- Fourier transform spectra of α-vanadyl phosphate found to be similar to that already reported [13].
3.4. TG/DTA/DTG of PVA, α -Vanadyl Phosphate
- Figure (4) shows the thermal decomposition of, α- VOPO4.2.5H2O. Thermal analysis was carried at the range 35-900℃. The loss of water of hydration occurs in two steps with two endothermic peaks at 125℃ and 175℃. Endothermic peak at 740℃ accompanied by weight loss equal to 3.12%. The total weight-loss found to be equal to 24.85%, calculated value 25.1%.
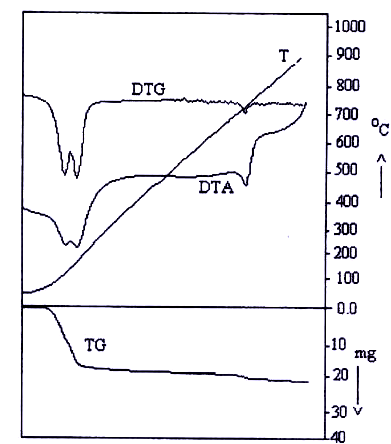 | Figure 4. TGA of α- VOPO4.2.5H2O |
3.5. Conductivity
- The conductivity (σ) α-vanadyl phosphate α- VOPO4.2.5H2O was found to be 1.74x10-4.Ω-1cm-1, 2.51x10-4 Ω-1cm-1, 7.17x10-4 Ω-1cm-1, at 25, 60 and 95℃, respectively. This material could be classified, according to literature [17, 18], as semiconductor or fast ionic conductor since the value of the conductivity is the order of liquid electrolytes with negligible electronic conductivity. Semiconductors have been attracted considerably due to their potential applications in various electrochemical devices, such as, solid state batteries, sensors, timers, fuel cells, memories, capacitors, etc. The conductivity (σ) was calculated using the equation.
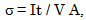 | (2) |
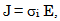 | (3) |
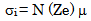 | (4) |
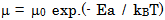 | (5) |
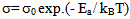 | (6) |
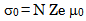 | (7) |
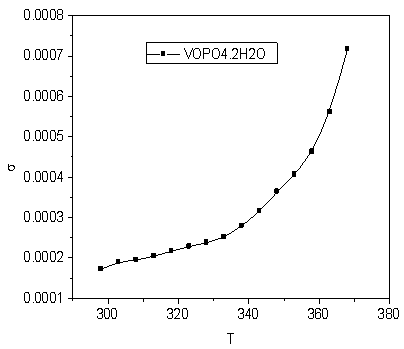 | Figure 5. The variation of the conductivity (σ) of α- VOPO4 2.5H2O with the absolute temperature |
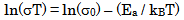 | (8) |
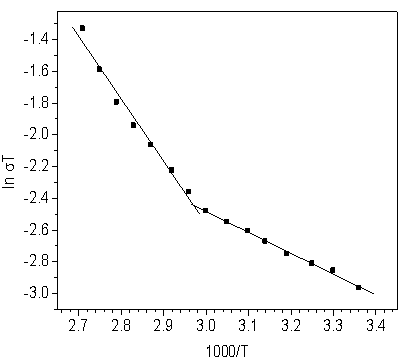 | Figure 6. Ln (σT) of α-VOPO4 2.5H2O as a function of inverse temperature |
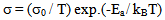 | (9) |
4. Conclusions
- In the present work, Layered α-vanadyl phosphate, α- VOPO4.2.5H2O, was prepared and characterized The conductivity (σ) was determined at the temperature range (25 - 95℃) and were found to be 1.74x10-4 Ω-1 cm-1, 2.51x 10-4 Ω-1cm-1 and 7.17x10-4 Ω-1 cm-1 at 25, 60 and 95℃, respectively. The conductivity of α- VOPO4.2.5H2O at room temperature (25℃) can be considered mixed protonic- electronic conductor. However; in the temperature range (60 - 95℃) the electronic conductance is enhanced by the presence of water molecules on its sites. The activation energy was calculated for the two temperature regions (25 – 60℃) and (65 -95℃), and found to be 0.113 and 0.339 eV, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML