-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(1): 59-67
doi:10.5923/j.chemistry.20140401.10
Volumetric Properties of Binary Systems of Tetrahydrofuran with Isomeric Butanols between 303.15 and 323.15 K
M. Kamrul Hossain 1, M. Abdur Rahaman 2, M. Shahadat Hossain 3, Shamima Aktar 1, Shamim Akhtar 4
1Department of Basic Science, World University of Bangladesh, Dhaka-1205, Bangladesh
2Department of Chemistry, Mawlana Bhashani Science and Technology University, Tangail-1902, Bangladesh
3Department of Chemistry, Comilla University, Comilla-3503, Bangladesh
4Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh
Correspondence to: Shamim Akhtar , Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Densities, 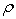 , have been measured for the binary systems of tetrahydrofuran + n-butanol, + sec-butanol and + tert-butanol in the whole range of composition between 303.15 and 323.15 K at an interval of 5 K. From measured
, have been measured for the binary systems of tetrahydrofuran + n-butanol, + sec-butanol and + tert-butanol in the whole range of composition between 303.15 and 323.15 K at an interval of 5 K. From measured 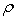 , excess molar volumes,
, excess molar volumes, 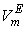 , apparent molal volumes,
, apparent molal volumes, 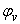 , thermal expansivities,
, thermal expansivities, 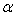 , and excess thermal expansivities,
, and excess thermal expansivities, 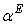 are estimated. For all systems, measured
are estimated. For all systems, measured 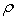 ,
, 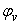 and
and 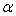 values are fitted to polynomial equations, whereas,
values are fitted to polynomial equations, whereas, 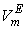 and
and 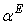 are fitted to Redlich-Kister equations. Here, the
are fitted to Redlich-Kister equations. Here, the 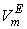 values are all positive but for tetrahydrofuran + n-butanol system, the values are small and for latter two systems, the values are large.
values are all positive but for tetrahydrofuran + n-butanol system, the values are small and for latter two systems, the values are large.
Keywords: Tetrahydrofuran, Molar volume, Isomeric butanols, Thermal expansivity
Cite this paper: M. Kamrul Hossain , M. Abdur Rahaman , M. Shahadat Hossain , Shamima Aktar , Shamim Akhtar , Volumetric Properties of Binary Systems of Tetrahydrofuran with Isomeric Butanols between 303.15 and 323.15 K, American Journal of Chemistry, Vol. 4 No. 1, 2014, pp. 59-67. doi: 10.5923/j.chemistry.20140401.10.
1. Introduction
- Tetrahydrofuran is a cyclic molecule, which is frequently utilized as a solvent in many pharmaceutical synthetic procedures because of its broad solvency for polar and non-polar compounds and it is excellent solvents of polymers and their application in polymer synthesis[1]. Alkanols are of interest because they serve as a simple example of biologically and industrially important amphiphilic materials[2]. Among them, butanol isomers are excellent solvents that find use as intermediates in polymerization and other chemical reactions and as cleaning agents for polymer surfaces and electronic materials. Binary systems containing oxygenated compounds, such as, ethers (-O-group) and alkanols (-0H group) are found to be of increasing applications, as these may be used as additives to gasolines, owing to their octane-enriching and pollution- reducing properties[3, 4]. As a continuation of alkanol + ether systems, therefore, the present investigation comprises of three sytems involving isomeric butanol with tetrahydrofuran. The choice of these butanols is simple, any variation in studied properties may be correlated to the effect of chain branching and/or the sterical hindrance effect due to increased -CH3 groups. A good number of literature survey shows that some systematic studies with composition and temperature dependences are still scarce. This paper reports the experimental density results for binary mixtures of tetrahydrofuran + n-butanol, + sec-butanol, + tert-butanol over the whole range of compositions at five temperatures from (303.15 to 323.15) K at atmospheric pressure.
2. Experimental Section
|
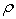 / g.cm-3) of experimental chemicals that were used to prepare the present binary systems at different temperatures and reported data are tabulated in Table 1. All the liquids were procured from Aldrich Chemical Co. Ltd. with quoted purities tetrahydrofuran (THF): 99.9%, n-butanol (NBA): 99.8%, sec-butanol (SBA): 99% and + tert-butanol (TBA): 99.5%. Densities were measured by using a 10 cm3 bi-capillary pycnometer. All weighing were made on a College B 204-S, Mettler Toledo digital balance with an accuracy of ±0.0001g. A thermostatically controlled water bath, capable of maintaining the temperature constant up to ± 0.05 K was used in the studies. The mole fraction is found to be accurate up to 10-4, while the uncertainty in the measured density is estimated as ±1.2×10-4 g cm-3.In order to find deviations from ideal behavior, excess molar volumes,
/ g.cm-3) of experimental chemicals that were used to prepare the present binary systems at different temperatures and reported data are tabulated in Table 1. All the liquids were procured from Aldrich Chemical Co. Ltd. with quoted purities tetrahydrofuran (THF): 99.9%, n-butanol (NBA): 99.8%, sec-butanol (SBA): 99% and + tert-butanol (TBA): 99.5%. Densities were measured by using a 10 cm3 bi-capillary pycnometer. All weighing were made on a College B 204-S, Mettler Toledo digital balance with an accuracy of ±0.0001g. A thermostatically controlled water bath, capable of maintaining the temperature constant up to ± 0.05 K was used in the studies. The mole fraction is found to be accurate up to 10-4, while the uncertainty in the measured density is estimated as ±1.2×10-4 g cm-3.In order to find deviations from ideal behavior, excess molar volumes, 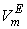 , for all systems were calculated from measured density data by using the given equation:
, for all systems were calculated from measured density data by using the given equation: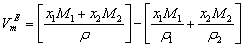 | (1) |
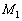 &
& 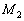 represent the molar masses,
represent the molar masses, 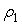 and
and 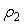 the densities,
the densities, 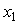 and
and 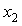 are the mole fractions of components 1 & 2, respectively and
are the mole fractions of components 1 & 2, respectively and 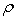 is the density of the mixture at the respective composition. Introducing molal concentration scale replacing mole fraction by molality from the measured density of the solvent, solute and solution the apparent molal volume were determined by following equation (2) and (3) for components 1 and 2.For component 1,
is the density of the mixture at the respective composition. Introducing molal concentration scale replacing mole fraction by molality from the measured density of the solvent, solute and solution the apparent molal volume were determined by following equation (2) and (3) for components 1 and 2.For component 1,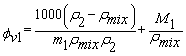 | (2) |
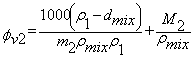 | (3) |
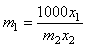 and
and 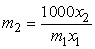 molality of component 1 and 2 respectively.The average isobaric thermal expansivity,
molality of component 1 and 2 respectively.The average isobaric thermal expansivity, 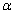 , was calculated as:
, was calculated as: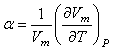 | (4) |
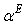 of a solution was then estimated by following the equation[25–26]:
of a solution was then estimated by following the equation[25–26]: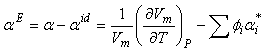 | (5) |
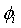 is the volume fraction,
is the volume fraction, 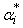 is thermal expansivity of the pure component i and the rest of the terms have their usual significances. All the estimated excess properties such as excess molar volume,
is thermal expansivity of the pure component i and the rest of the terms have their usual significances. All the estimated excess properties such as excess molar volume, 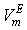 , for a system as a function of composition are fitted to Redlich-Kister equation[27]:
, for a system as a function of composition are fitted to Redlich-Kister equation[27]: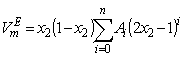 | (6) |
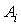 is the fitting coefficient and
is the fitting coefficient and 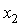 is the mole fraction of the aromatic hydrocarbons. The standard deviation,
is the mole fraction of the aromatic hydrocarbons. The standard deviation, 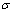 , followed the equation:
, followed the equation: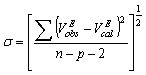 | (7) |
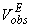 and
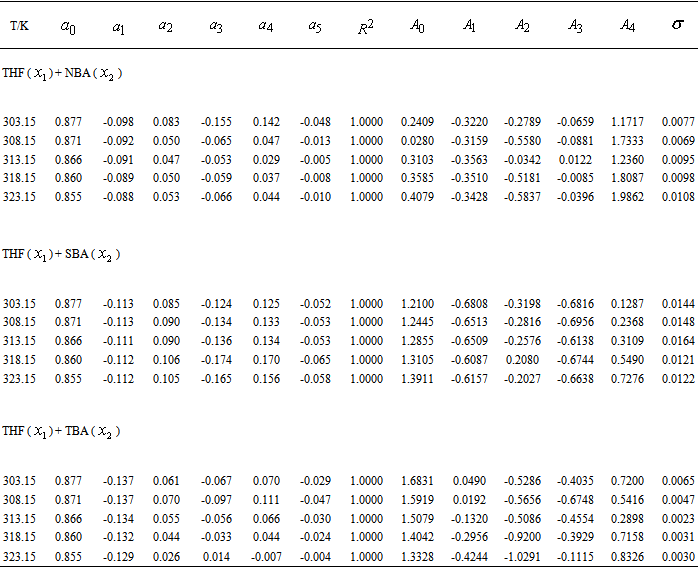
and 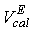 are the observed and calculated excess molar volumes, n the total number of compositions for a particular system and p is the number of coefficients are as shown in Table 4.
are the observed and calculated excess molar volumes, n the total number of compositions for a particular system and p is the number of coefficients are as shown in Table 4.3. Results and Discussion
- Densities (
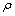 ) of tetrahydrofuran (THF) + n-butanol (NBA), + sec-butanol (SBA) and + tert-butanol (TBA) in the whole range of composition (0 ≤
) of tetrahydrofuran (THF) + n-butanol (NBA), + sec-butanol (SBA) and + tert-butanol (TBA) in the whole range of composition (0 ≤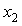 ≤ 1), where,
≤ 1), where, 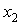 represents the mole fraction of the butanol under consideration, measured at an interval of 5 K between 303.15 and 323.15 K are summarized in Table 2. At a particular temperature the
represents the mole fraction of the butanol under consideration, measured at an interval of 5 K between 303.15 and 323.15 K are summarized in Table 2. At a particular temperature the 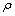 values were fitted by a five-degree polynomial equation of the form:
values were fitted by a five-degree polynomial equation of the form: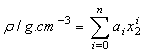 | (8) |
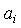 and relevant
and relevant 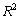 are tabulated in Table 3. Densities of the pure butanols are found to follow the order: NBA > SBA > TBA and for the systems follow the same general trend, i.e.,
are tabulated in Table 3. Densities of the pure butanols are found to follow the order: NBA > SBA > TBA and for the systems follow the same general trend, i.e., 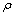 decreases gradually with the increment of
decreases gradually with the increment of 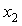 for all the THF + NBA, THF + SBA and THF + TBA systems. In alcohols, though the proton accepting capacity increases in the order tertiary > secondary > primary, H-bonding capacity increases in the reverse order. As it is found the fraction of free -OH group is more in isomeric forms than in n-alkanols[28]. That means, NBA, SBA may favour to form more extensive H-bonded networks compared to TBA. Tertiary butanol (TBA) so forms a weaker H-bonding and hence, it is less associated. This is as manifested by the higher density values of NBA and SBA compared to TBA.
for all the THF + NBA, THF + SBA and THF + TBA systems. In alcohols, though the proton accepting capacity increases in the order tertiary > secondary > primary, H-bonding capacity increases in the reverse order. As it is found the fraction of free -OH group is more in isomeric forms than in n-alkanols[28]. That means, NBA, SBA may favour to form more extensive H-bonded networks compared to TBA. Tertiary butanol (TBA) so forms a weaker H-bonding and hence, it is less associated. This is as manifested by the higher density values of NBA and SBA compared to TBA.
|
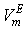 of the three systems against
of the three systems against 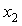 at 303.15 and 323.15 K. For each system,
at 303.15 and 323.15 K. For each system, 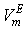 at different temperatures have been fitted well by Redlich-Kister equation[27] and the relevant fitting coefficients being shown in Table 3. In THF + NBA system, as the values are small the variation of
at different temperatures have been fitted well by Redlich-Kister equation[27] and the relevant fitting coefficients being shown in Table 3. In THF + NBA system, as the values are small the variation of 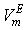 against
against 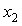 are not so smooth. Moreover,
are not so smooth. Moreover, 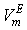 forms a small maximum in the NBA-rich region near
forms a small maximum in the NBA-rich region near 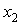 = 0.9. On the other hand,
= 0.9. On the other hand, 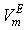 vs.
vs. 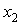 forms a large positive lobes with maximum at
forms a large positive lobes with maximum at 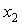 ~ 0.4 and 0.5 for THF + SBA and + TBA systems, respectively. The above behaviors can be explained as follows. It may be stated that, addition of small amount of NBA in THF initially involves complete rupture of O-H--O type bonding in NBA, together with dispersion in THF. But as fast as monomeric NBA species are formed, intermolecular interaction due to dipole-dipole and/or H-bonding between NBA and THF molecules may also occur, contributing negatively to the
~ 0.4 and 0.5 for THF + SBA and + TBA systems, respectively. The above behaviors can be explained as follows. It may be stated that, addition of small amount of NBA in THF initially involves complete rupture of O-H--O type bonding in NBA, together with dispersion in THF. But as fast as monomeric NBA species are formed, intermolecular interaction due to dipole-dipole and/or H-bonding between NBA and THF molecules may also occur, contributing negatively to the 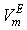 . However, the former factors outweigh the later ones, so that, the overall
. However, the former factors outweigh the later ones, so that, the overall 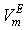 though small, is positive for the THF + NBA. Similar results of positive VmE in the three systems were also observed previously by Alonso et al[29], Brocos[30] and Aminabhavi et al[31]. With the increase in alkanol concentration (
though small, is positive for the THF + NBA. Similar results of positive VmE in the three systems were also observed previously by Alonso et al[29], Brocos[30] and Aminabhavi et al[31]. With the increase in alkanol concentration (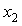 ), NBA molecules undergo restructuring through H-bonding as well as dipole-dipole interactions among themselves. As a result positive
), NBA molecules undergo restructuring through H-bonding as well as dipole-dipole interactions among themselves. As a result positive 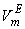 values start to decrease correspondingly.Again, when each of SBA and TBA to THF, destruction of SBA-SBA and TBA-TBA H-bonding occurs more easily due to the steric hindrance effect through increasing –CH3 groups compared to NBA and subsequently all the monomeric or segregated species are more dispersed weakening the dipole-dipole interaction in THF further. As a result, in THF + SBA and THF+TBA systems, factors that cause volume expansion seem to exert greater influence over those of volume contraction at all temperatures over the whole range of composition. Again the comparative diagram of Fig. 1 shows that,
values start to decrease correspondingly.Again, when each of SBA and TBA to THF, destruction of SBA-SBA and TBA-TBA H-bonding occurs more easily due to the steric hindrance effect through increasing –CH3 groups compared to NBA and subsequently all the monomeric or segregated species are more dispersed weakening the dipole-dipole interaction in THF further. As a result, in THF + SBA and THF+TBA systems, factors that cause volume expansion seem to exert greater influence over those of volume contraction at all temperatures over the whole range of composition. Again the comparative diagram of Fig. 1 shows that, 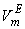 values vary as THF + TBA > THF + SBA > THF+NBA. With the rise of temperature, structure-breaking processes dominate over the structure making interactions. So that, in the whole range of composition,
values vary as THF + TBA > THF + SBA > THF+NBA. With the rise of temperature, structure-breaking processes dominate over the structure making interactions. So that, in the whole range of composition, 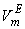 , values increase proportionately leading to positive
, values increase proportionately leading to positive 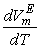 for the THF + NBA system.The apparent molal volumes,
for the THF + NBA system.The apparent molal volumes, 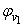 and
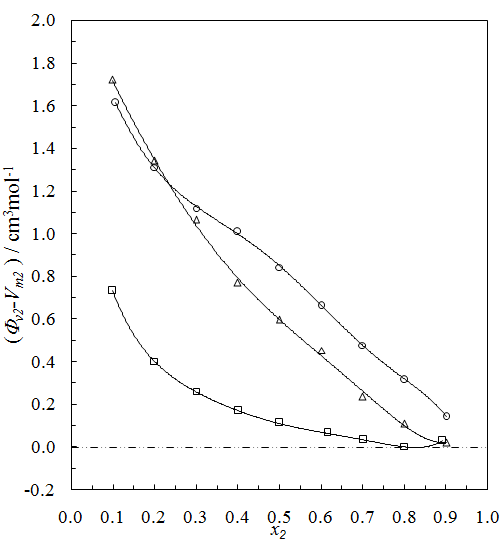
and 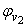 of component 1 and 2, in their binary mixtures as calculated by the equations (2) and (3) respectively, and the difference between apparent molal volume and molar volume of each of components 1 and 2, i.e.; (
of component 1 and 2, in their binary mixtures as calculated by the equations (2) and (3) respectively, and the difference between apparent molal volume and molar volume of each of components 1 and 2, i.e.; (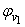 -
- 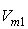 ) and (
) and (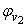 -
- 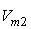 ) were estimated at 303.15 K and graphically represented by Figs. 2-3. For both the components, these differences are positive indicating the dominance of dispersive forces almost over all compositions.
) were estimated at 303.15 K and graphically represented by Figs. 2-3. For both the components, these differences are positive indicating the dominance of dispersive forces almost over all compositions.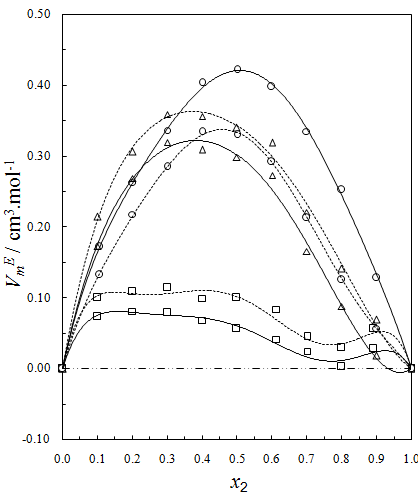 | Figure 1. Comparison of excess molar volumes,  , for the systems of , for the systems of  + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K (—) and 323.15 K (---) + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K (—) and 323.15 K (---) |
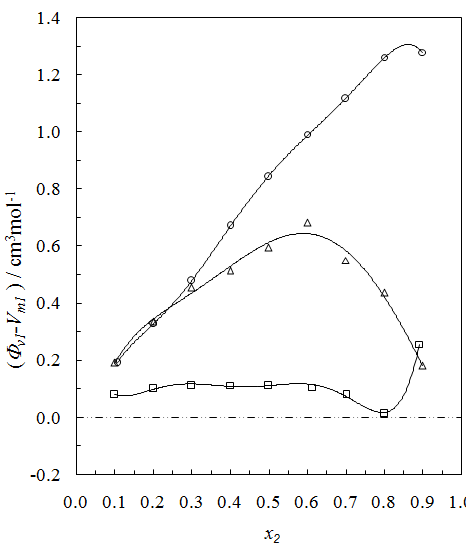 | Figure 2. Plot of (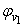 - - 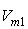 ) of component 1 in the systems of ) of component 1 in the systems of 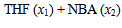 (□), + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K (□), + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K |
 | Figure 3. Plot of (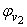 - - 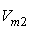 ) of component 2 in the systems of ) of component 2 in the systems of 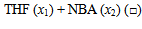 , + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K , + SBA (x2) (Δ) and + TBA (x2) (o) at 303.15 K |
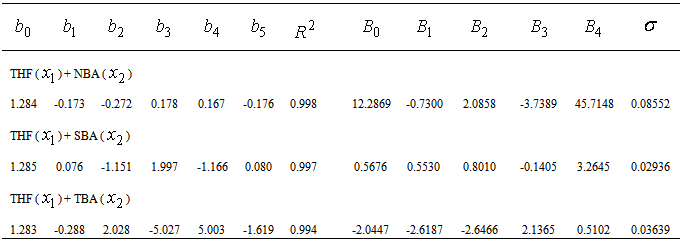
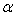 , and its excess value,
, and its excess value, 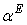 , estimated by equation (4) & (5) respectively; the relevant coefficients being summarized as in Table 5. Figs. 4 and 5 indicate
, estimated by equation (4) & (5) respectively; the relevant coefficients being summarized as in Table 5. Figs. 4 and 5 indicate 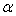 and
and 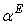 of the three systems against
of the three systems against 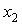 .
. 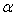 of THF + NBA and THF + SBA decreases with the increase of alcohols in the whole range of concentration, but in THF + TBA it largely increases in the alcohol rich region and follow the order: THF + TBA > + SBA > + NBA. On the other hand,
of THF + NBA and THF + SBA decreases with the increase of alcohols in the whole range of concentration, but in THF + TBA it largely increases in the alcohol rich region and follow the order: THF + TBA > + SBA > + NBA. On the other hand, 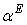 values are positive for THF + NBA and THF + SBA systems, but negative for THF + TBA system. Positive
values are positive for THF + NBA and THF + SBA systems, but negative for THF + TBA system. Positive 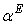 signifies that structure breaking effects are more at high temperatures, whereas, under similar conditions structure making effects seem to be favorable in the THF + TBA system.
signifies that structure breaking effects are more at high temperatures, whereas, under similar conditions structure making effects seem to be favorable in the THF + TBA system.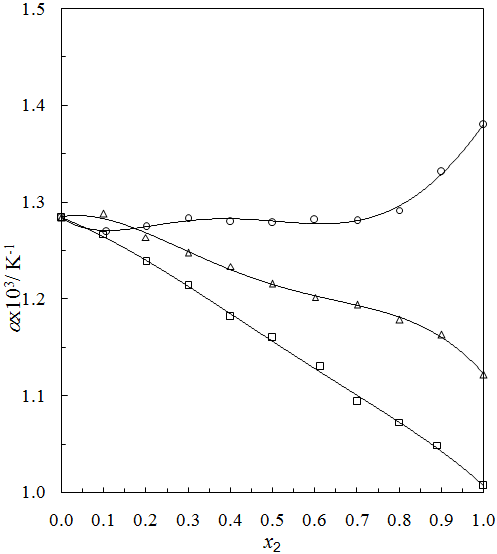 | Figure 4. Plot of thermal expansivities, 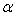 , of the systems of THF (x1) + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) , of the systems of THF (x1) + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) |
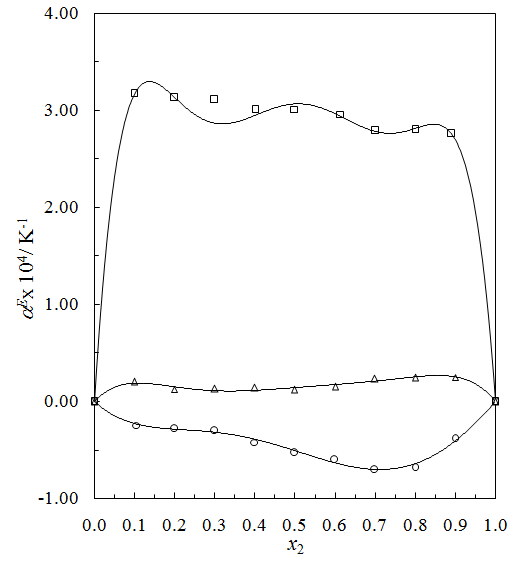 | Figure 5. Plot of excess thermal expansivities, 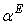 , of the systems of , of the systems of 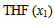 + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) + NBA (x2) (□), + SBA (x2) (Δ) and + TBA (x2) (o) |
|
|
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the financial grant from the Ministry of Science, Information and Communication Technology, Government of the People's Republic of Bangladesh, for the project “Physical Properties and Molecular Interactions in Liquid Systems”.
References
| [1] | Marsh K.N., Niamskul P., Gmehling J., Bolts R., 1999, Review of thermophysical property measurements on mixtures containing MTBE, TAME, and other ethers with non-polar solvents, Fluid Phase Equilib. 156, 207–227. |
| [2] | Cerderinna, C. A., Tovar C. A., Carballo, E., Romnai, L., Delgado, M. C., Torres, L. A., Costas, M., 2002, Temperature dependence of the excess molar heat capacities for alcohol−alkane mixtures. Experimental testing of the predictions from a Two-State Model. J. Phys. Chem. B, 106, 185−191. |
| [3] | Brandrup J., Immergut E.H., Polymer Handbook, 3rd ed., A Wiley Interscience, Publications, 1989. |
| [4] | Csikos, R., Pallay J., Radchenko E.D., Englin B.A., Robert, J.A., 1976, Hydrocarbon process. Int. Ed., 55, 21. |
| [5] | Aralaguppi M.I., Jadar C.V., and Aminabhavi T.M., 1996, Density, refractive Index, viscosity, and speed of sound in binary mixtures of 2-ethoxyethanol with dioxane, acetonitrile, and tetrahydrofuran at (298.15, 303.15, and 308.15) K, J. Chem. Eng. Data, 41, 1307. |
| [6] | Muhuri P.K., Das, B., Hazra D.K., 1996, Viscosities and excess molar volumes of binary mixtures of propylene carbonate with tetrahydrofuran and methanol at different temperatures, J.Chem. Eng. Data, 41(6), 1473-1476, 1996. |
| [7] | Suri S.K. 1981. Excess volumes of binary mixtures of tetrahydrofuran with various hydrocarbons at 313.15 K., Can. J. Chem., 59(19), 2839-2844. |
| [8] | Saleh, M.A., Akhtar S., Ahmed M.S. and Uddin, M.H., 2002, Excess molar volumes and thermal expansivities of aqueous solutions of dimethylsulfoxide, tetrahydrofuran and 1,4-dioxane, Phys. Chem. Liq., 40(5), 621–635. |
| [9] | Leppla C., Rezanova E.N. , and Lichtenthaler R.N., 2000, Thermodynamic excess properties for ternary mixtures 1-butanol + (butyl vinyl ether or isobutyl vinyl ether) + heptane, J. Chem. Eng. Data, 45 (6), 1019 -1026. |
| [10] | Troncoso J., Valencia J.L., Souto-Caride M., González-Salgado D. and Peleteiro J., 2004, Liquid−Liquid equilibria for the ternary systems of (water + tetrahydrofuran + polar Solvent) at 298.15 K, J. Chem. Eng. Data, 49 (6), 1827 -1832. |
| [11] | Cibulka, 1993, Saturated liquid densities of 1-alkanols from C1 to c10 and n-alkanes from C5 to C16: A critical evaluation of experimental data, Fluid Phase equilibria, 89, 1-18. |
| [12] | Troncoso J., Valencia J.L., Souto-Caride M., González-Salgado D., and Peleteiro J., 2004, Thermodynamic properties of dodecane + 1-butanol and + 2-butanol systems, J. Chem. Eng. Data, 14, 1789–1793. |
| [13] | Lintomen L., Pinto R.T.P., Batista E., Meirelles A.J.A. and Maciel M.R.W., 2000, Liquid−Liquid equilibrium of the water + citric acid + 2-butanol + sodium chloride system at 298.15 K , J. Chem. Eng. Data, 45 (6), 1211–1214. |
| [14] | Wen-Lu W., Liang-Tau C., I-Min S.; 2000, Densities and viscosities for binary mixtures of butylamine with aliphatic alcohols, J. Chem. Eng. Data, 45 (4), 606–609. |
| [15] | Wieczorek, S.A., 1984, Determination of the excess molar volumes of (butan-2-ol + n-heptane) between 293.15 and 308.15 K by use of a continuous dilution dilatometer, J. Chem. Thermodynamics, 16,115-119. |
| [16] | Nikam P.S., Mahale T.R., and Hasan M., 1996, Density and viscosity of binary mixtures of ethyl acetate with methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, 2-methylpropan-1-ol, and 2-methylpropan-2-ol at (298.15, 303.15, and 308.15) K, J. Chem. Eng. Data, 1996, 41 (5), 1055–1058. |
| [17] | Kim E.S. and MarshK.N., 1988, Excess volumes for 2-methyl-2-propanol + water at 5 K intervals from 303.15 to 323.15 K, J. Chem. Eng. Data, 33 (3), 288–292. |
| [18] | Kipkemboi P.K. and Easteal A.J., 1994, Densities and viscosities of binary aqueous mixtures of nonelectrolytes: tert-butyl alcohol and tert-butylamine, Can. J. Chem., 72(9), 1937-1945. |
| [19] | Anson A., Garriga R., Martinez S., Perez P. and Gracia M., 2005, Densities and viscosities of binary mixtures of 1-bromobutane with butanol Isomers at several temperatures, J. Chem. Eng. Data, 50, 1478–1483. |
| [20] | Fattahi M., Iloukhani H., 2010, Excess molar volume, viscosity, refractive index study for the ternary mixture {2-methyl-2-butanol (1) + tetrahydrofuran (2), + propylamin (3) at different temperatures. Application of the ERAS-model and Peng-Robinson-Stryjek-Vera equation of state. J. Chem. Thermodynamics, 42, 1335-1345. |
| [21] | Pal A., Kumar H., Kumar B., Gaba R., 2013, Density and speed of sound for binary mixtures of 1,4-dioxane with propanol and butanol isomers at different temperatures, J. Mol. Liquids, 187, 278-286. |
| [22] | Bravo-Sanchez M., Iglesias-Silva G. A., Estrada-Baltazar, A., Hall, K. R., 2010, Densities and viscosities of binary mixtures of n-butanol with 2-butanol, isobutanol, and tert-butanol from (303.15 to 343.15) K. J. Chem. Eng. Data, 55, 2310−2315. |
| [23] | Gupta M., Vibhu I., Shukla J. P., 2006, Ultrasonic velocity, viscosity and excess properties of binary mixture of tetrahydrofuran with 1-propanol and 2-propanol, Fluid Phase Equilib. 244,26–32. |
| [24] | Gadzuric S., Nikolic A., Vranes M., Jovic B., Damjanovic M., Dozic S., 2012, Volumetric properties of binary mixtures of N-ethylformamide with tetrahydrofuran, 2-butanone, and ethylacetate from T = (293.15 to 313.15) K, J. Chem. Thermodynamics 51, 37–44. |
| [25] | Kiyohara O., D'Arcy P.J., Benson G.C., 1978, Thermal expansivities of water + tetrahydrofuran mixtures at 298.15K., Can. J. Chem., 56(22) 2803-2807. |
| [26] | G.C. Benson G.C., Kiyohara O., 1979, Evaluation of excess isentropic compressibilities and isochoric heat capacities, J. Chem. Thermodyn. 11, 1061– 1064. |
| [27] | Redlich O.J., Kister A.T., 1948, Algebraic representation of thermodynamic properties and the classification of solutions, Ind. Eng. Chem. 40, 345–348. |
| [28] | Pikkarainnen; L., 1983, Densities and viscosities of binary mixtures of N,N-dimethylacetamide with aliphatic alcohols, J. Chem. Eng. Data, 28 (3), 344–347. |
| [29] | Alonso V., Calvo E., Bravo R., Pintos M., Amigo A., 1994, Thermodynamic properties of tetrahydropyran + 1-alkanol Mixtures,J. Chem. Eng. Data, 39 (4), 926–928. |
| [30] | Brocos P., Calvo E., Piñeiro A., Bravo R., Amigo A., Roux A.H. and Roux-Desgranges G., 1999, Heat capacities, excess enthalpies, and volumes of mixtures containing cyclic ethers. 5. Binary Systems {1,3-Dioxolane + 1-Alkanols}, J. Chem. Eng. Data, 44 (6), 1341–1347. |
| [31] | Aminabhavi T.M., Gopalkrishna B., 1994, Densities, viscosities, and refractive indices of the binary mixtures of bis(2-methoxyethyl) ether with 1-propanol, 1-butanol, 2-methyl-1-propanol, and 2-methyl-2-propanol, J. Chem. Eng. Data, 39 (4), 865–867. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML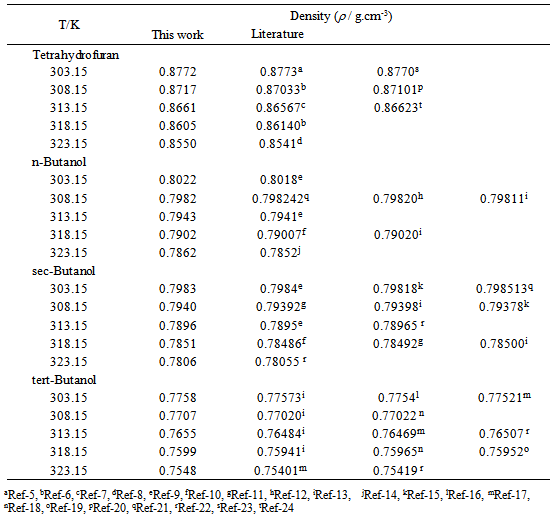
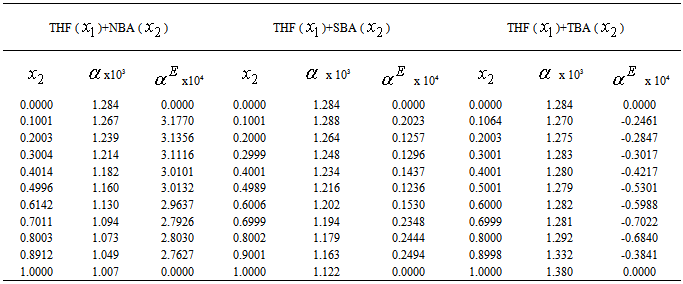
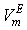 (cm3.mol-1) of the systems of THF (x1) + NBA (x2), + SBA (x2) and + TBA (x2) for different molar ratios at different temperatures
(cm3.mol-1) of the systems of THF (x1) + NBA (x2), + SBA (x2) and + TBA (x2) for different molar ratios at different temperatures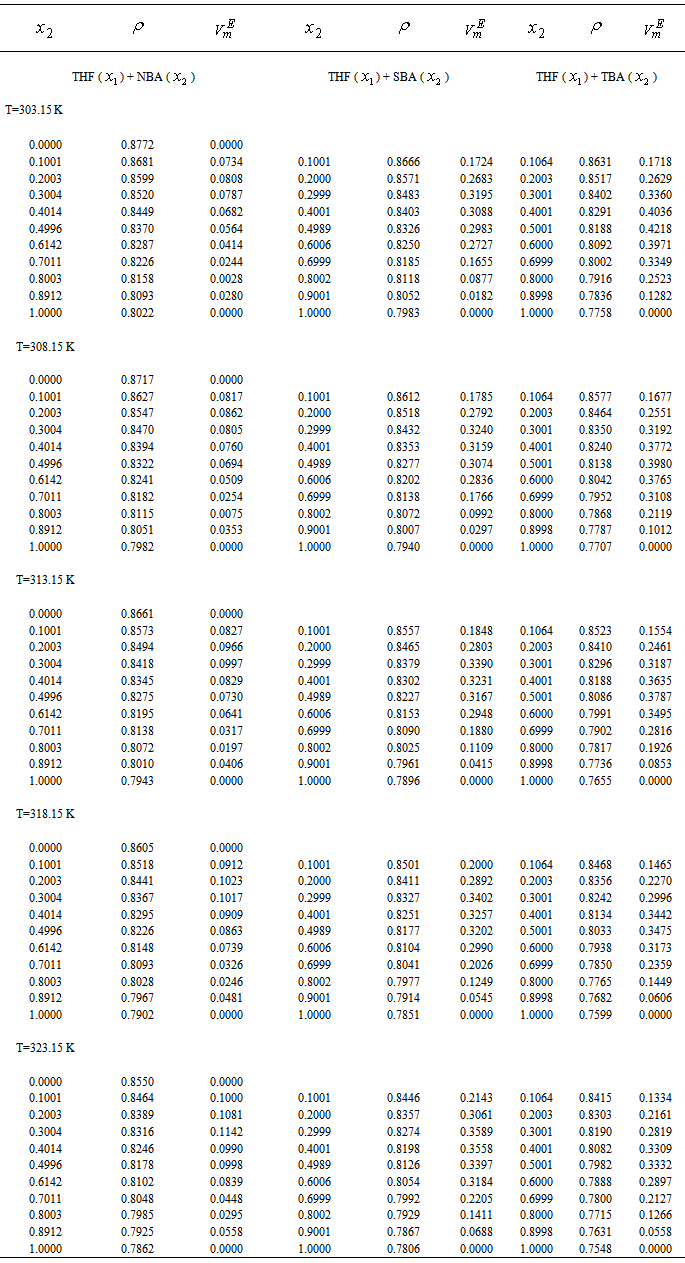
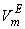 (cm3.mol-1) of the systems of THF (x1) + NBA (x2), + SBA (x2) and + TBA (x2) at different temperatures
(cm3.mol-1) of the systems of THF (x1) + NBA (x2), + SBA (x2) and + TBA (x2) at different temperatures