-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(1): 29-34
doi:10.5923/j.chemistry.20140401.04
Multi-component Approach for the Synthesis of Fused Dihydropyridines via Unsymmetrical Hantzch Condensation Using Glycerol as Green Solvent
Harvinder Singh Sohal1, Rajshree Khare1, Arun Goyal1, Andrew Woolley2, Kishanpal Singh3, Rajeev Sharma4
1Department of Chemistry, Maharishi Markandeshwar University, Mullana-133 207, Haryana, India
2Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON, M5S 3H6, Canada
3Department of Chemistry, Punjabi University, Patiala-147 001, Punjab, India
4Department of Chemistry, Multani Mal Modi College, Patiala-147 001, Punjab, India
Correspondence to: Harvinder Singh Sohal, Department of Chemistry, Maharishi Markandeshwar University, Mullana-133 207, Haryana, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Some fused 1,4-dihydropyridine derivatives have been synthesized in one-pot, multi-component condensation of dimedone, aldehyde, malononitrile and ammonium acetate using glycerol, an eco-friendly, readily available green solvent which costs virtually nothing. The targeted molecules are obtained in a yield, which is excellent, to say the least. The synthesized compounds were purified by simple recrystallisation.
Keywords: Glycerol, Hantzch Condensation, 1,4-Dihyropyridine, Multi-component, Dimedone
Cite this paper: Harvinder Singh Sohal, Rajshree Khare, Arun Goyal, Andrew Woolley, Kishanpal Singh, Rajeev Sharma, Multi-component Approach for the Synthesis of Fused Dihydropyridines via Unsymmetrical Hantzch Condensation Using Glycerol as Green Solvent, American Journal of Chemistry, Vol. 4 No. 1, 2014, pp. 29-34. doi: 10.5923/j.chemistry.20140401.04.
Article Outline
1. Introduction
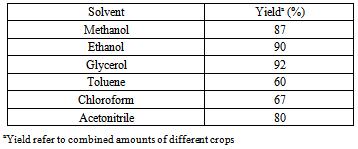
- The chemistry of 1,4-dihydropyridines (1,4-DHPs) began in 1882 with Hantzsch condensation[1]. Since then 1,4-dihydropyridine ring has caught the attention due to its link with the biological system in the form of coenzyme, diphosphopyridine nucleotide (DPNH)[2] and also found as a bio-active material. The 1,4-dihydropyridines are an important class of Ca2+ channel blockers and are also known to be effective cardiovascular agents for the treatment of hypertension. Apart from these activities, 1,4-DHPs are found to be as PAF-acether antagonists [3], calcium antagonists[4], antihypertensives[5], cerebral antischemic activity in the treatment of Alzheimer’s disease and chemosensitizer acting in tumor therapy. Presently, number of 1,4-dihydropyridine based drugs have been commercialised such as nifedipine, felodipine[6], nicardipine[7], amlodipine[8] and many more have appeared in the market[9]. On the other hand, green solvents and solvent from renewable resources has gained much interest in the recent years[10]. Use of water has attracted much attention[11] but water based processes are still subject to limitations due to solubility problems of highly hydrophobic substrates. However, excellent solvent properties like low toxicity [LD50 (oral rat) 12600 mg/kg], biodegradability, low-flammability, long liquid range (boiling point 290℃), low vapour pressure and solubility of polar organic compounds has made the glycerol an excellent option to use as solvent for organic synthesis. Further with the present emphasis and increasing demand of biodiesel, which is responsible for the excess production of glycerol as by-product, triggered the discovery of processes that use glycerol for the synthesis of value added chemicals, as reaction medium and for other applications[12, 13]. Recently glycerol has been used for Heck and Suzuki coupling[14], Michael addition[15], Fridel-Crafts type addition, epoxide ring opening[16], synthesis of xanthenes[17] and very recently for the production of benzodiazepines and octahydroacridines[18] etc. Understanding the importance of 1,4-DHPs, plethora of reports on the synthesis of 1,4-DHPs and modifications of the original Hantzch reaction appeared in the literature[19-26]. But some of the reported protocols have one or another drawback like collection and purification of catalyst that may cause harmful effects, harsh reaction conditions, unsatisfactory yields, use of harmful solvents, etc. Persisting with our work on heterocyclics[27], it is high time when we develop a procedure, which is pro-environment, clean and economical. In the present report, glycerol mediated unsymmetrical hantzch reaction have been reported (Scheme 1).
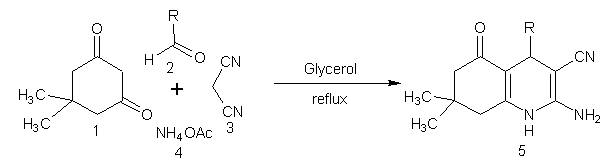 | Scheme 1. |
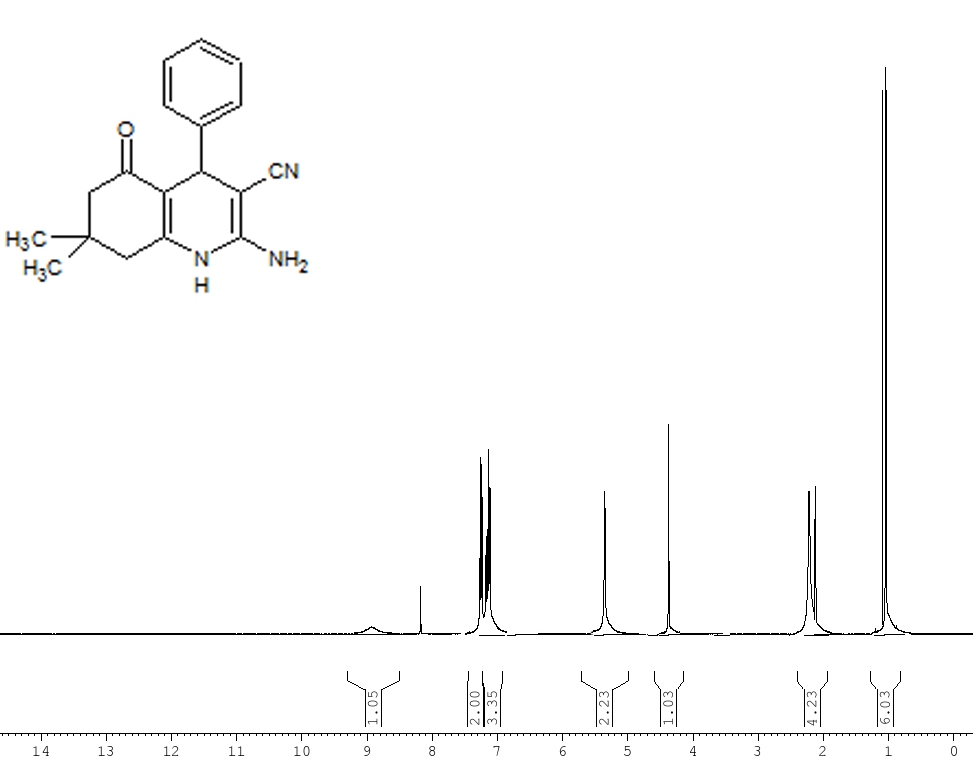 | Figure 1. 1H NMR of the 2-Amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline 5a |
2. Result and Discussion
|
|
|
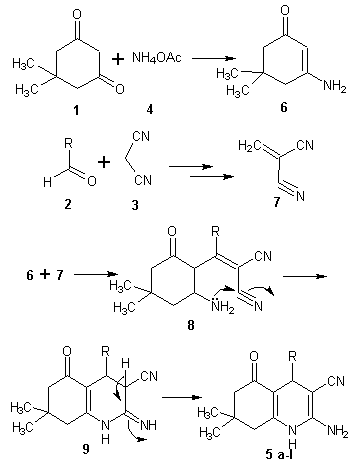
3. Conclusions
- The present procedure is an effective method for production of highly functionalized 1,4-dihydropyridine from readily available starting materials in a single step with inherent flexibility and diversity. Another advantage of this method is minimization of time, labor, cost, less waste production and devoid of harsh reaction conditions.
4. Experimental
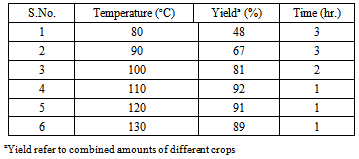
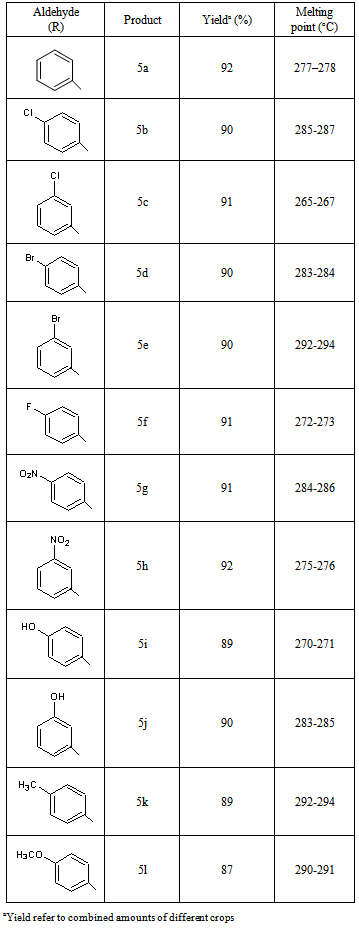
- Materials were obtained from commercial suppliers and were used without further purifications. Melting points were recorded in open end capillaries and are uncorrected. 1H NMR spectras were recorded in CDCl3 solution on a Bruker Avance II 400 MHz spectrometer; chemical shifts (delta) are reported in ppm relative to TMS as internal standard. The IR spectras were obtained on a Perkin-Elmer 237B spectrometer.Synthesis of2-Amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline 5a: In a conical flask benzaldehyde (0.01 mol), dimedone (0.01 mol), ethyl malononitrile (0.01 mol), ammonium acetate (0.02 mol) and glycerol (10 ml) were taken and heated at 120℃ for the stipulated time table 2. After the completion of reaction (vide TLC), reaction mixture was cooled to room temperature and added 50 ml ice-cold water when solid separated out. Filtered and dried, recrystalised from ethanol to afford compound 5a, 92% yield, mp 277-278 oC (entry 1, Table 3). Similarly, other aldehydes 3 b-l were reacted with dimedone, malononitrile and ammonium acetate to afford various 1,4-dihydropyridines derivatives 5 b-l (Table 3).Spectral data of some selected compounds:2-Amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5a): mp. 277–278 °C; IR (KBr): ΰ = 3436, 3324, 3214, 2197, 1719, 1498 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.02 (s, 3H, CH3), 1.09 (s, 3H, CH3), 2.01–2.37 (m, 4H, 2×CH2), 4.38 (s, 1H, CH), 5.36 (s, 2H, NH2), 7.07–7.29 (m, 5H, ArH), 8.94 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.4, 29.5, 32.8, 36.9, 39.4, 50.8, 59.7, 113.5, 119.7, 126.2, 127.8, 128.4, 143.9, 155.3, 166.3, 197.7. MS (EI) m/z 294.3 (M+). Anal. Calcd for C18H19N3O: C, 73.72; H, 6.48; N, 14.33. Found: C, 73.61; H, 6.58; N, 14.31.2-Amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5b): mp. 285-287°C; IR (KBr): ΰ = 3437, 3326, 3218, 2188, 1722, 1505 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.97 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.02–2.35 (m, 4H, 2×CH2), 4.17 (s, 1H, CH), 5.48 (s, 2H, NH2), 7.08–7.19 (m, 4H, ArH), 8.99 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 26.8, 29.9, 32.5, 36.7, 39.8, 51.4, 59.3, 113.5, 120.8, 126.6, 127.7, 128.4, 145.5, 155.6, 166.7, 194.8. MS (EI) m/z 327.45 (M+). Anal. Calcd for C18H18ClN3O: C, 65.96; H, 5.50; N, 12.83. Found:C, 65.87; H, 5.44; N, 12.82.2-Amino-4-(3-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5c): mp. 265-267°C; IR (KBr): ΰ = 3429, 3328, 3227, 2197, 1720, 1505 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.03 (s, 3H, CH3), 1.09 (s, 3H, CH3), 1.97–2.38 (m, 4H, 2×CH2), 4.34 (s, 1H, CH), 5.39 (s, 2H, NH2), 7.21–7.41 (m, 4H, ArH), 8.96 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ = 27.4, 29.7, 32.7, 36.6, 39.8, 51.4, 59.5, 113.4, 120.8, 126.9, 127.4, 128.5, 143.6, 155.7, 167.5, 193.8. MS (EI) m/z 327.45 (M+). Anal. Calcd for C18H18ClN3O: C, 65.96; H, 5.50; N, 12.83. Found: C, 65.95; H, 5.48; N, 12.83.2-Amino-4-(4-bromophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5d): mp. 283-284°C; IR (KBr): ΰ = 3435, 3336, 3223, 2196, 1721, 1504 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.97 (s, 3H, CH3), 1.05 (s, 3H, CH3), 2.05–2.35 (m, 4H, 2×CH2), 4.36 (s, 1H, CH), 5.46 (s, 2H, NH2), 7.02–7.27 (m, 4H, ArH), 8.83 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.4, 29.6, 32.7, 36.5, 39.5, 50.9, 59.7, 112.9, 119.7, 126.5, 127.5, 128.7, 144.5, 155.6, 169.0, 192.6. MS (EI) m/z 371.9 (M+). Anal. Calcd for C18H18BrN3O: C, 58.08; H, 4.84; N, 11.29. Found: C, 58.02; H, 4.80; N, 11.25.2-Amino-4-(3-bromophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5e): mp. 292-294°C; IR (KBr): ΰ = 3428, 3326, 3216, 2197, 1722, 1513 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.94 (s, 3H, CH3), 0.98 (s, 3H, CH3), 2.14–2.47 (m, 4H, 2×CH2), 4.36 (s, 1H, CH), 5.37 (s, 2H, NH2), 7.09–7.21 (m, 4H, ArH), 8.90 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.6, 29.3, 32.6, 36.7, 39.4, 50.9, 59.6, 113.5, 120.6, 126.3, 127.4, 128.7, 144.3, 155.5, 166.8, 196.9. MS (EI) m/z 371.9 (M+). Anal. Calcd for C18H18BrN3O: C, 58.08; H, 4.84; N, 11.28. Found: C, 58.03; H, 4.81; N, 11.31.2-Amino-4-(4-fluorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5f): mp. 272-273°C; IR (KBr): ΰ = 3441, 3334, 3213, 2184, 1721, 1503 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.01 (s, 3H, CH3), 1.07 (s, 3H, CH3), 2.11–2.49 (m, 4H, 2×CH2), 4.22 (s, 1H, CH), 5.52 (s, 2H, NH2), 7.16–7.32 (m, 4H, ArH), 8.84 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.4, 30.1, 32.4, 36.5, 39.7, 50.8, 59.8, 113.5, 121.6, 127.4, 127.5, 128.3, 144.5, 155.7, 167.9, 196.3 ppm; MS (EI) m/z 312.35 (M+). Anal. Calcd for C18H18FN3O: C, 69.44; H, 5.83; N, 13.50. Found: C, 69.40; H, 5.83; N, 13.49.2-Amino-4-(4-nitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5g): mp. 284-286°C; IR (KBr): ΰ = 3434, 3325, 3219, 2209, 1725, 1504 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.07 (s, 3H, CH3), 1.09 (s, 3H, CH3), 2.07–2.49 (m, 4H, 2×CH2), 4.28 (s, 1H, CH), 5.53 (s, 2H, NH2), 7.22–7.37 (m, 4H, ArH), 8.90 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 26.9, 29.9, 33.0, 37.2, 39.7, 51.5, 59.7, 113.7, 121.1, 127.2, 127.2, 128.5, 144.6, 154.5, 167.6, 195.8. MS (EI) m/z 338 (M+). Anal. Calcd for C18H18N4O3: C, 63.90; H, 5.32; N, 16.56. Found: C, 63.77; H, 5.32; N, 16.48.2-Amino-4-(3-nitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (5h): mp. 275-276°C; IR (KBr): 3423, 3325, 3216, 2196, 1722, 1507 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.07 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.20–2.56 (m, 4H, 2×CH2), 4.59 (s, 1H, CH), 4.77 (s, 2H, NH2), 7.48–7.75 (m, 4H, ArH), 8.92 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.3, 30.4, 32.8, 36.9, 39.9, 51.1, 59.6, 112.8, 120.6, 126.8, 127.1, 128.5, 144.6, 155.2, 168.2, 195.4. MS (EI) m/z 338 (M+). Anal. Calcd for C18H18N4O3: C, 63.90; H, 5.32; N, 16.56. Found: C, 63.81; H, 5.30; N, 16.51.2-Amino-4-(4-hydroxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5i): mp. 270-271°C; IR (KBr): ΰ = 3436, 3384, 3326, 3204, 2196, 1719, 1497 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 1.05 (s, 3H, CH3), 1.09 (s, 3H, CH3), 2.11–2.45 (m, 4H, 2×CH2), 4.36 (s, 1H, CH), 5.89 (s, 2H, NH2), 7.07–7.29 (m, 4H, ArH), 8.78 (s, 1H, NH), 9.76 (s, 1H, OH). 13C NMR (100 MHz, CDCl3): δ = 26.8, 29.7, 32.7, 36.8, 39.7, 50.6, 59.9, 113.4, 120.8, 126.5, 127.6, 128.2, 144.3, 154.7, 166.7, 194.9. MS (EI) m/z 309 (M+). Anal. Calcd for C18H19N3O2: C, 69.90; H, 6.15; N, 13.59. Found: C, 69.83; H, 6.13; N, 13.58.2-Amino-4-(3-hydroxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5j): mp. 283-285°C; IR (KBr): ΰ = 3429, 3383, 3337, 3224, 2202, 1717, 1504 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.95 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.99–2.30 (m, 4H, 2×CH2), 4.29 (s, 1H, CH), 5.47 (s, 2H, NH2), 7.18–7.29 (m, 4H, ArH), 8.88 (s, 1H, NH), 9.81 (s, 1H, OH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 26.9, 29.8, 32.5, 36.6, 39.6, 50.7, 59.6, 113.5, 121.4, 126.6, 127.7, 128.5, 143.8, 158.0, 166.4, 195.8. MS (EI) m/z 309 (M+). Anal. Calcd for C18H19N3O2: C, 69.90; H, 6.15; N, 13.59. Found: C, 69.77; H, 6.14; N, 13.55.2-Amino-4-(4-methylphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5k): mp. 292-294°C; IR (KBr,): 3437, 3320, 3222, 2213, 1718, 1495 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.97 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.04 (s, 3H, CH3), 1.99–2.36 (m, 4H, 2×CH2), 4.47 (s, 1H, CH), 5.45 (s, 2H, NH2), 7.02–7.19 (m, 4H, ArH), 8.79 (s, 1H, NH) ppm; 13C NMR (100 MHz, CDCl3): δ(ppm) = 27.2, 30.3, 32.9, 36.2, 39.2, 50.8, 59.9, 113.5, 121.7, 126.3, 127.6, 128.7, 144.5, 156.7, 168.6, 195.9. MS (EI) m/z 307 (M+). Anal. Calcd for C19H21N3O: C, 74.27; H, 6.84; N, 13.68. Found: C, 74.19; H, 6.81; N, 13.62.2-Amino-4-(4-methoxylphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(5l): mp. 290-291°C; IR (KBr): ΰ = 3446, 3329, 3223, 2209, 1717, 1499 cm−1. 1H NMR (400 MHz, CDCl3): δ(ppm) = 0.95 (s, 3H, CH3), 1.02 (s, 3H, CH3), 3.65 (s, 3H, OCH3), 2.10–2.48 (m, 4H, 2×CH2), 4.28 (s, 1H, CH), 5.57 (s, 2H, NH2), 7.17–7.39 (m, 4H, ArH), 8.84 (s, 1H, NH). 13C NMR (100 MHz, CDCl3): δ(ppm) = 26.7, 29.5, 32.7, 37.4, 39.5, 51.3, 59.2, 114.3, 120.8, 126.9, 127.4, 128.7, 144.5, 156.8, 168.3, 195.8. MS (EI) m/z 323 (M+). Anal. Calcd for C19H21N3O2: C, 70.58; H, 6.50; N, 13.00. Found: C, 70.45; H, 6.41; N, 13.01.
ACKNOWLEDGEMENTS
- The authors thank M. M. University, Mullana, Ambala, India for the financial support and Harvinder Singh Sohal and Arun Goyal also thank Mr. Vikas Pahwa for the liberal support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML