-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(1): 22-28
doi:10.5923/j.chemistry.20140401.03
Synthesis and Thermal Behavior of Novel Pillared Ө-Type Zirconium Phosphate 1,10-Phenanthroline Zn(II), Cd(II), Cr(III), Fe(III) and La(III) Materials
Sadek Shakshooki, Bashir Elnageh Ali, Samia El-Rais, Mahmood El-Rais
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: Sadek Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ө-Type zirconium phosphate, Zr(HPO4)21.88H2O, was prepared and characterized. Reaction of 0.1M 1,10-phenanthroline in ethanol with Ө-typeZr(HPO4)21.88H2O, lead to the formation of Ө-typeZr(HPO4)2 (Phen)0.2760.5H2O. Novel pillared materials: Ө-Zr(H)1.1(PO4)2(Phen)0.276(Zn)0.451.1H2O, Ө-Zr(H)1.95(Phen)0.276(PO4)2(Cd)0.051 1.1H2O, Ө-Zr(H)0.5(PO4)2(Phen)0.25(Cr)0.50.45H2O, Ө-Zr(H)0.8(PO4)2(Phen)0.275(Fe)0.41.43H2O, Ө-Zr(H)0.82(PO4)2(Phen)0.275 (La)0.391.3H2O, were prepared and characterized by chemical, X-ray diffraction, thermal analysis and FT-IR spectroscopy. Their metal ions contents were determined. The pillared materials show the increase of their thermal stability which can be related to metal ions effect. These materials can be considered as new solid acid catalysts, inorganic ion exchangers and as ionic conductance materials.
Keywords: Ө-Type Zirconium Phosphate, Pillared Ө- type Zirconium Phosphate - 1,10-phenanthroline
Cite this paper: Sadek Shakshooki, Bashir Elnageh Ali, Samia El-Rais, Mahmood El-Rais, Synthesis and Thermal Behavior of Novel Pillared Ө-Type Zirconium Phosphate 1,10-Phenanthroline Zn(II), Cd(II), Cr(III), Fe(III) and La(III) Materials, American Journal of Chemistry, Vol. 4 No. 1, 2014, pp. 22-28. doi: 10.5923/j.chemistry.20140401.03.
Article Outline
1. Introduction
- Tetravalent metal phosphates are very insoluble compounds with good thermal stabilities and high ion exchange capacities[1,2]. The discovery of their crystalline materials[3,4], represent a fundamental step in chemistry of these compounds with general formula α-M(IV) (HPO4)2 H2O, and γ-M(IV)PO4H2PO42H2O, (where M = Ti, Zr, Hf, Ge, Sn , Ce). These materials contain structural POH groups with labile protons. They can exchange their protons with counter ions such as alkali, alkaline earth, transition divalent and trivalent metal ions[1-4] and act as intercalates[1,2 5,6]. Increase attention direct toward their intercalation[5,6], catalytic[7], electrical conductance[8], and sensors[9]. Layered zirconium phosphates have potential applications as inorganic fillers, sorpents and solid acid catalysts[14-16].This class of compounds can bond themselves to pillaring reactions by metal amine complexes exchanged in their interlayer. Successful pillaring of these type of layered materials can accomplished via amine intercalation reaction [17-21]. They can form complex pillars between the layers as its formation in-situ by ion exchange of transition metal ions and ligand intercalation. As catalysts attracted attention recently and still in their infancy[22-24]. However, to date very little work on pillared materials of layered zirconium and titanium phosphates. In our Laboratory we are carrying out systematic studies on novel lamellar M(IV) phosphates Here we are reporting synthesis and thermal properties of novel of Ө-typezirconium phosphate 1,10-phenanthroline -Zn(II), Cd(II), Cr(III), Fe(III) and La(III) pillared materials.
2. Materials and Methods
- Chemicals: ZrOCl28H2O,H3PO4(85%),CdCl26H2O,1,10-Phenanthroline, HF, HCl (purchased from BDH), Zn(NO3)24H2O, Cr(NO3)3 3H2O (purchased from LTD), Fe(NO3)39H2O (purchased from ERBA) ,NaOH, NaCl (T- Baker), La(NO3)36H2O (purchased from Riedel-de Haen) were used as received. Other reagents used were of analytical grade.Instruments used for characterization§ X-ray powder Diffractometer PW/1710 Philips, using Ni-filtered CuKα (λ= 1.5405Å).§ Nexus 670 FT-IR Nicolet spectrophotometer.§ Derivatograph MOM-C Budapest, Hungary and Shimadzu TGA-60H TG/DTA.§ Atomic Absorption Spectrophotometer Alpha4 (SPEX).§ Perkin-Elmer 2400 automatic elemental analyzer§ pH Meter WGW 521.Preparation of Ө-type zirconium phosphate 50ml 0.5M ZrOCl28H2O in 3M HF were mixed with 200ml of (4.6M) H3PO4 in Pyrex round bottom flask (prior to mixing, the solutions were cooled at ~15ºC). The mixture was left at ~15ºC for 3days. The resultant precipitate was washed with distilled water, by addition and decantation of distilled water up to pH3.The resultant product was filtered and dried in air for 72 hrs at room temperature ~25ºC.Preparation of Ө- type zirconium phosphate- 1,10- phenanthroline110 ml of 0.1M 1,10 phenanthroline in ethanol were added to 1.5g Ө-type zirconium phosphate, Zr(HPO4)2 1.88H2O, with stirring at room temperature. After complete addition the stirring was continued for 5days at room temperature and 5hrs at 50ºC. The resultant product was filtered, washed with ethanol and dried in air for 48hrs.Preparation of Ө-type zirconium phosphate-1,10- phenanthrolineZn(II)To 100mg of Ө-type zirconium phosphate1,10– phenanthroline, 3.5 ml of 0.05 M Zn(NO3)24H2O in HNO3 solution of pH4 solution were added, followed by addition of 3.5 ml of distilled water with stirring at room temperature. The stirring continued for 24hrs at room temperature (~25ºC). The product was filtered , washed with distilled water, an dried in air for 48hrs. The filtrate plus water washing were collected and diluted up to 100 ml.Preparation of pillared Ө-type zirconium phosphate -1,10- phenanthrolineCd(II) To 100mg of Ө–type zirconium phosphate-1,10- phenanthroline, 3.5 ml of 0.05M CdCl26H2O in HNO3 solution of pH4 solution were added, followed by addition of 3.5ml of distilled water, with stirring, at room temperature. The stirring was continued for 24 hrs, at room temperature , the product was filtered washed with distilled water and dried in air for 48 hrs. The filtrate plus water washing were collected and diluted up to 100 ml.Preparation of pillared Ө-typezirconium phosphate 1,10-phenanthroline Cr(III)To 100mg of Ө- type zirconium phosphate 6 ml of 0.05 M Cr(NO3)33H2O in HNO3 solution of pH4 were added with stirring at room temperature. The stirring was continued for 24hrs. The product was filtered washed with distilled water and dried in air for 48hrs. The filtrate and water washing were collected and diluted up to 100 ml.Preparation of pillared Ө-type zirconium phosphate -1,10-phenanthroline Fe(III)To 100mg of Ө-type zirconium phosphate -1,10 phenanthroline 6 ml of 0.05 M Fe(NO3)3.9H2O in HNO3 solution of pH4 were added with stirring at room temperature. The stirring was continued for 24hrs. The product was filtered washed with distilled water and dried in air for 48hrs. The filtrate and water washing were collected and diluted to 100ml.The above mentioned filtrates plus water washing were kept for atomic absorption analysis for the remaining Zn2+, Cd2+, Cr3+, Fe3+ metal ions.Preparation of pillared Ө-type zirconium phosphate -1,10-phenanthrolineLa(III)To 100mg Ө-type zirconium phosphate -1,10- phenanthroline, 6 ml of 0.05 M La(NO3)3.6H2O in HNO3 solution of pH4 were added with stirring at room temperature .The stirring was continued for 24hrs at room temperature. The product was filtered washed with distilled water and dried in air for 48hrs. The filtrate and water washing were collected diluted to 100 ml.( kept for analysis of La3+ ions by EDTA method).
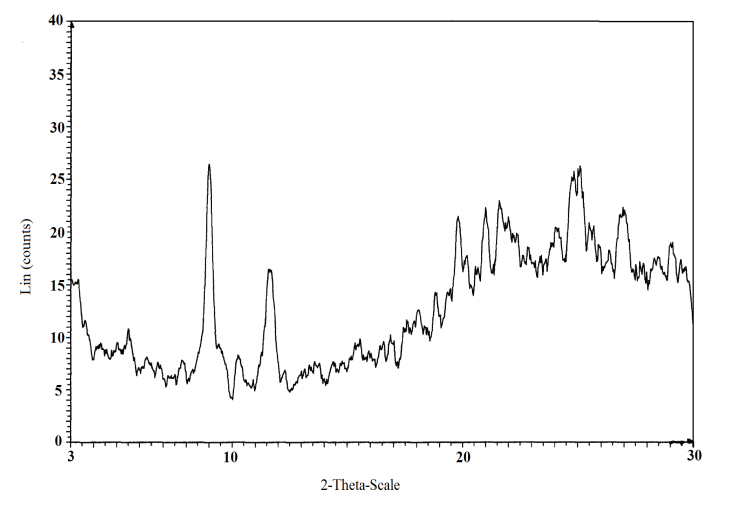 | Figure 1. X-ray diffractogram of Ө-type Zr(HPO4)21.88H2O |
3. Results and Discussions
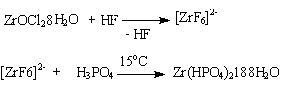
- θ-Type zirconium phosphate, Ө-Zr(HPO4)2.1.88H2O, was prepared from reaction of tetravalent zirconium salt and H3PO4 in HF solution. The reaction can be described as:
 The resultant product was characterized by chemical, X-ray and thermal analysis and by FT-IR spectroscopy. Its exchange capacity was determined by Na+ ions titration.XRDFigure 1 shows the X-ray powder diffraction pattern of the θ-type zirconium phosphate, shows the presence of diffraction maxima with basal spacing equal 9.85Å. The Ө-type materials exhibit lamellar structure. Negatively charged layers are formed by macroanions [Mn(IV)(HPO4)2]2- and protons (H+) bonded to the oxygen adjacent to the anionic layer form positively charged layers. The water molecules occupying crystallographic sites are located almost in the center of interlayer cavities.FT-IRFT-IR becomes a key tool to investigate structure of tetravalent metal phosphates[1,2,22].Figure 2 Shows FT-IR spectrum of Ө-type zirconium phosphate in the range 4000-400cm-1 wave number. The narrow bands at 3604.65, 3434.11cm-1 and band at 1640.39cm-1 are assigned to vibrational modes of H2O molecules, suggest that water molecules are located at well defined crystallographic sites. These bands at 3434.11, 3434.11cm-1 were also attributed to O-H asymmetric modes of interlayer water molecules. The band at 1640.39cm-1 also corresponds to H-O-H bending modes. The broad band at 3147.10cm-1 assigned to (P)OH stretching mode of the hydrogen bond, it had shoulder at 3310cm-1 attributed to O-H stretching coming from symmetry lowering effect of the H2O interlayer molecules. The bands at the region 1273.21-1054.46 cm-1 are assigned as P-O asymmetry stretching of PO4 groups, while a band at 976.33 cm-1 is characteristic to the bonding in plane of the (P-O) bond. The bands in the region 609.14 to 515.39cm-1 ascribed to the presence of δ(PO4) and to vibration of water molecules (609.14cm-1), while the band at 671.64 cm-1 is connected with O-H bond (out of plane). A tentative assignment of various vibration modes is proposed based on previous works preformed in other M(IV) phosphate compounds [1,2,22].
The resultant product was characterized by chemical, X-ray and thermal analysis and by FT-IR spectroscopy. Its exchange capacity was determined by Na+ ions titration.XRDFigure 1 shows the X-ray powder diffraction pattern of the θ-type zirconium phosphate, shows the presence of diffraction maxima with basal spacing equal 9.85Å. The Ө-type materials exhibit lamellar structure. Negatively charged layers are formed by macroanions [Mn(IV)(HPO4)2]2- and protons (H+) bonded to the oxygen adjacent to the anionic layer form positively charged layers. The water molecules occupying crystallographic sites are located almost in the center of interlayer cavities.FT-IRFT-IR becomes a key tool to investigate structure of tetravalent metal phosphates[1,2,22].Figure 2 Shows FT-IR spectrum of Ө-type zirconium phosphate in the range 4000-400cm-1 wave number. The narrow bands at 3604.65, 3434.11cm-1 and band at 1640.39cm-1 are assigned to vibrational modes of H2O molecules, suggest that water molecules are located at well defined crystallographic sites. These bands at 3434.11, 3434.11cm-1 were also attributed to O-H asymmetric modes of interlayer water molecules. The band at 1640.39cm-1 also corresponds to H-O-H bending modes. The broad band at 3147.10cm-1 assigned to (P)OH stretching mode of the hydrogen bond, it had shoulder at 3310cm-1 attributed to O-H stretching coming from symmetry lowering effect of the H2O interlayer molecules. The bands at the region 1273.21-1054.46 cm-1 are assigned as P-O asymmetry stretching of PO4 groups, while a band at 976.33 cm-1 is characteristic to the bonding in plane of the (P-O) bond. The bands in the region 609.14 to 515.39cm-1 ascribed to the presence of δ(PO4) and to vibration of water molecules (609.14cm-1), while the band at 671.64 cm-1 is connected with O-H bond (out of plane). A tentative assignment of various vibration modes is proposed based on previous works preformed in other M(IV) phosphate compounds [1,2,22].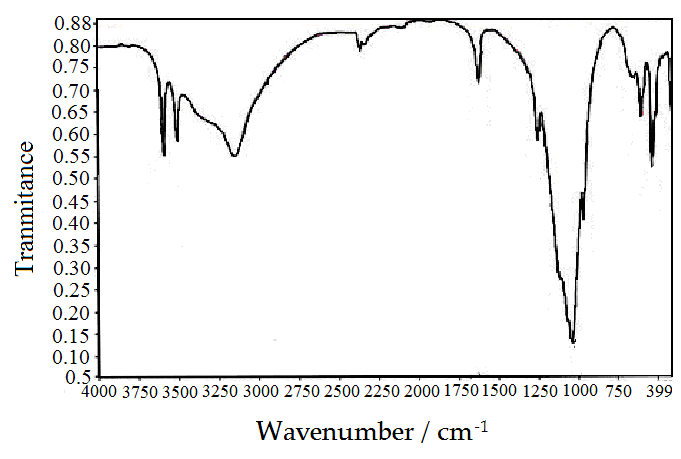 | Figure 2. FT- IR-spectra of Ө-type Zr(HPO4)21.88H2O |
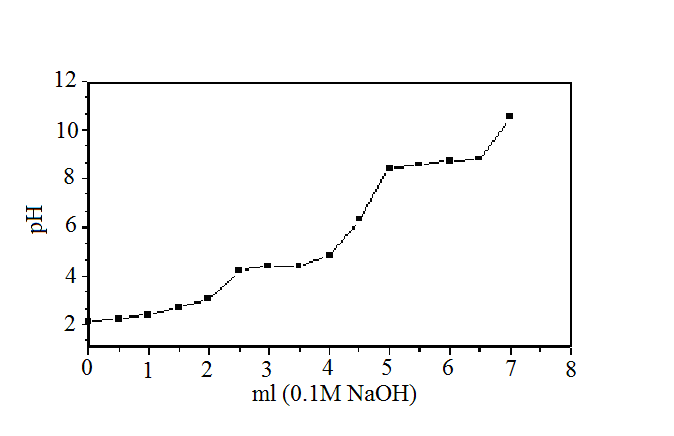 | Figure 3. Na+ ions titration curve of Ө- type of Zr(HPO4)21.88H2O. |
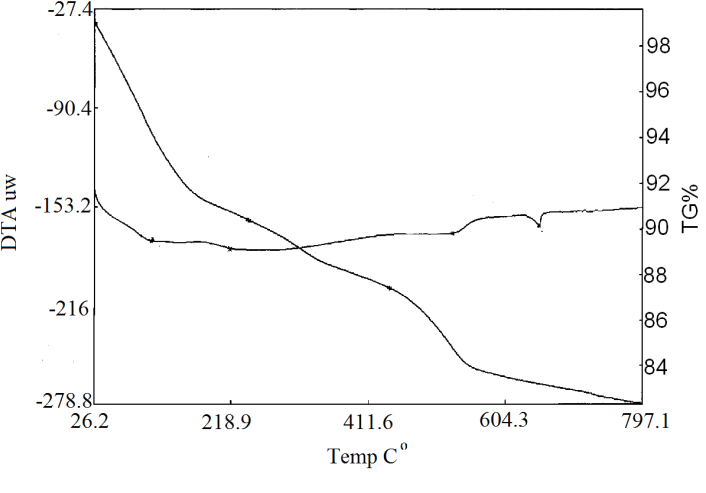 | Figure 4. TG/DTA of Ө-type of Zr(HPO4)21.88H2O |
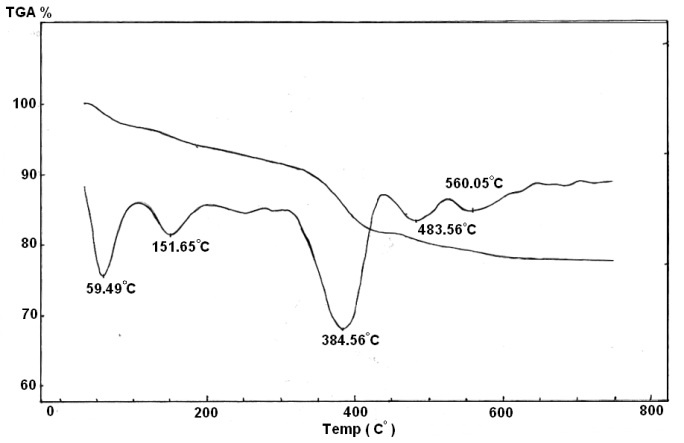 | Figure 5. TGA/DTA of Ө-Type Zr(HPO4)2(Phen)0.276 0.5H2O |
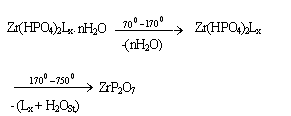 where nH2O = water of hydration, L = ,1,10-phenanthroline x = loading of the organic base, H2OSt = the structural water results from POH groups condensation. The thermal behavior of the materials found to follow the same trend of that observed for inclusion products of layered tetravalent metal phosphates[6,7,23,24]. The thermal decomposition of the organic ligand found to super imposed wih POH groups condensation . Thermal decomposition of pillared materialsThe thermal decomposition of the inclusion product can be described as follows :
where nH2O = water of hydration, L = ,1,10-phenanthroline x = loading of the organic base, H2OSt = the structural water results from POH groups condensation. The thermal behavior of the materials found to follow the same trend of that observed for inclusion products of layered tetravalent metal phosphates[6,7,23,24]. The thermal decomposition of the organic ligand found to super imposed wih POH groups condensation . Thermal decomposition of pillared materialsThe thermal decomposition of the inclusion product can be described as follows :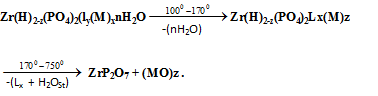 where nH2O = water of hydration, L = ,1,10-phenanthroline x = loading of the organic base, H2OSt = the structural water results from POH groups condensation, M the mtalion equivalen. The thermal behavior of the materials found to follow the same trend of that observed for pillared materials of layered tetravalent metal phosphates[6,7,23,24]. The thermal decomposition of the organic ligand found to superimpose with POH groups condensation and the oxidation of the metal ions. TG/DTA of pillared Ө-Type zirconium phosphate -1,10-phenanthroline Zn(II)
where nH2O = water of hydration, L = ,1,10-phenanthroline x = loading of the organic base, H2OSt = the structural water results from POH groups condensation, M the mtalion equivalen. The thermal behavior of the materials found to follow the same trend of that observed for pillared materials of layered tetravalent metal phosphates[6,7,23,24]. The thermal decomposition of the organic ligand found to superimpose with POH groups condensation and the oxidation of the metal ions. TG/DTA of pillared Ө-Type zirconium phosphate -1,10-phenanthroline Zn(II)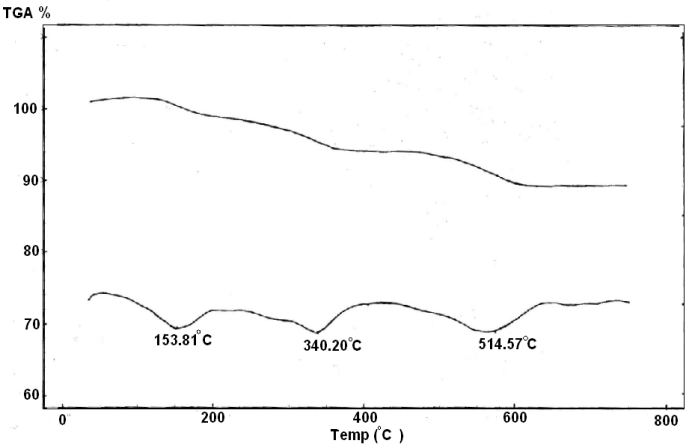 | Figure 6. TGA/DTA of Ө-type Zr(H)1.1(PO4)2(Phen)0.276 (Zn)0.451.1H2O |
 | Figure 7. TGA/DTA of Ө-type Zr(H)1.95(PO4)2(Phen)0.276 (Cd)0.051.1H2O |
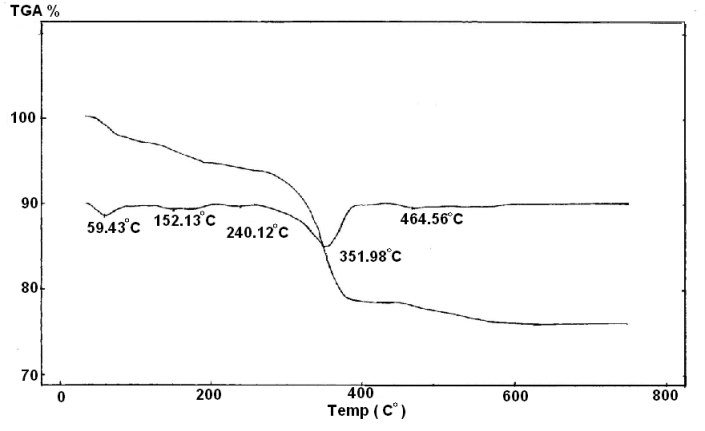
 | Figure 8. Figure 8 :TGA/DTA of Ө-type Zr(H)0.5(PO4)2(Phen)0.25(Cr)0.50.45H2O |
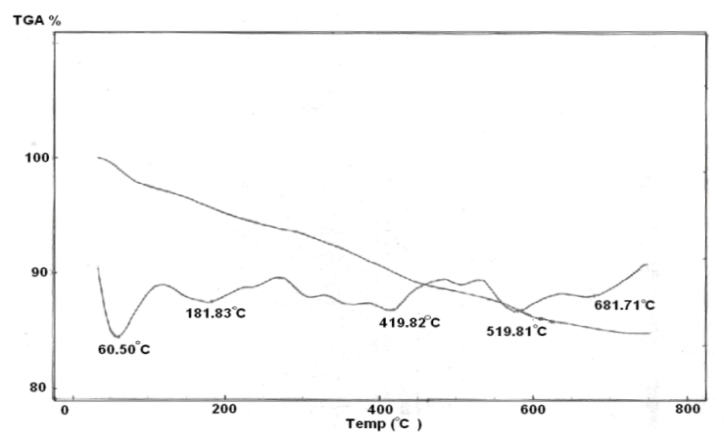
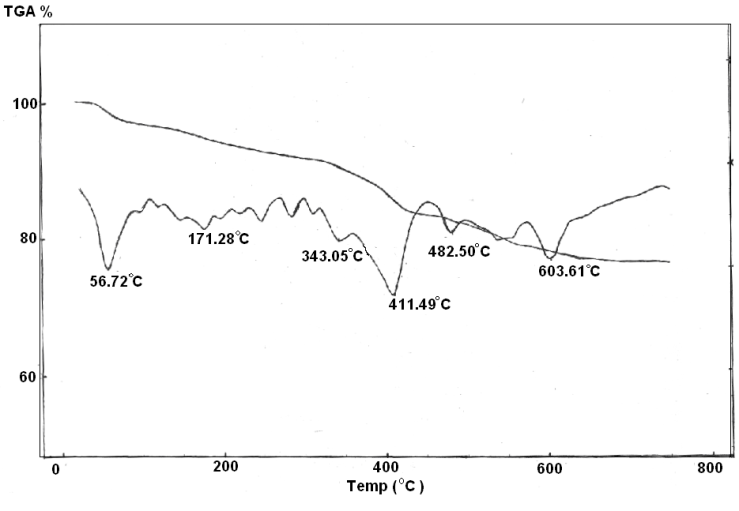 | Figure 9. TGA/TDA of Ө-Zr(H)0.8(PO4)2 (Phen)0.275(Fe)0.4.1.43H2O |
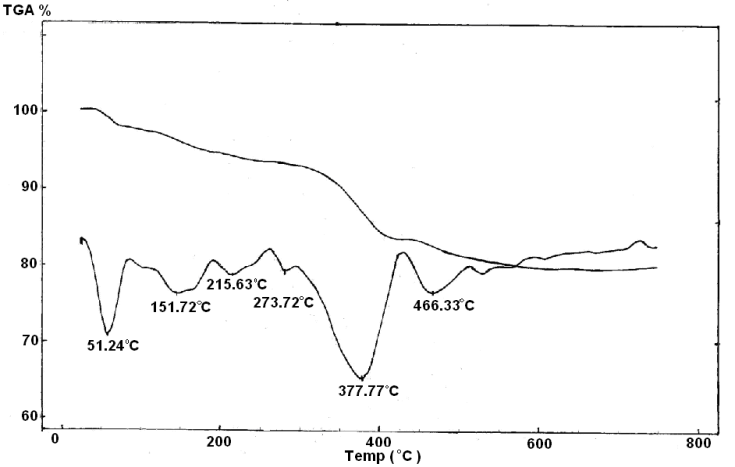 | Figure 10. TGA/TDA of Ө-Zr(H)0.82(PO4)2 (Phen)0.275(La)0.39.1.3H2O |
4. Conclusions
- This study shows that Ө-zirconium phosphates can be obtained by direct precipitation method, the HF method. The formulation of the investigated pillared of Ө-zirconium phosphate is based on a fact that M(IV) phosphates are stable materials and extremely insoluble. The final products from thermal treatment are the pyrophosphates and metal oxides. The pillars formed or when the Ө-zirconium phosphates was used as ion exchangers and intercalate material. i.e. in its counter ions adsorbed forms. The loading of the organic ligand found to be quite low from the direct intercalation of 1,10-phenanthroline., this is due to covering effect. The pillared materials show the increase of their thermal stability which can be related to metal ions effect. These materials can be considered as new solid acid catalysts, inorganic ion exchangers and as ionic conductance materials.
ACKNOWLEDGEMENTS
- To Departments of Chemistry, Faculty of Sciences, Tripoli University and Sebha University for providing research facilities, to microanalysis centre of Cairo University for elemental and TGA analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML