-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2014; 4(1): 10-21
doi:10.5923/j.chemistry.20140401.02
Synthesis, Characterization and Antimicrobial Properties of Mannich Base Cyclization Derivatives of Benzimidazole and Their Metal Complexes
Misbah Ur Rehman1, Muhammad Arif2, Muhammad Imran2, Muhammad Farooq3
1Institute of Chemical Science, Gomal University, D.I. Khan, KPK, 29050, Pakistan
2Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, 60800, Pakistan
3Department of Chemistry, Govt. College Gujranwala, 52250, Pakistan
Correspondence to: Muhammad Imran, Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, 60800, Pakistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Novel Mannich base cyclization derivatives of Benzimidazole were prepared through three-component condensation reaction of 2-aminobenzimidazole with formaldehyde and primary amines. All the compounds were characterized through spectral and analytical data. The transition metal complexes of resultant Mannich bases have been synthesized and well characterized by elemental analyses, spectral studies, magnetic moment determination, molar conductivity measurement and Thermogravimetric analysis. Experimental results showed that metal complexes act as bi-dentate ligands. The in-vitro antibacterial and antifungal activity of Mannich bases and their metal (II) complexes was assayed against different pathogens using MIC method. All the compounds and their metal complexes showed good potency against various microorganisms. The synthesized compounds and their metal complexes were also screened for their cytotoxicity and results showed that only Ni (II) complexes exhibit cytotoxicity while all other compounds were almost inactive.
Keywords: Mannich bases, Benzimidazole, Metal complexes, Anti-microbial agents
Cite this paper: Misbah Ur Rehman, Muhammad Arif, Muhammad Imran, Muhammad Farooq, Synthesis, Characterization and Antimicrobial Properties of Mannich Base Cyclization Derivatives of Benzimidazole and Their Metal Complexes, American Journal of Chemistry, Vol. 4 No. 1, 2014, pp. 10-21. doi: 10.5923/j.chemistry.20140401.02.
Article Outline
- 2.4. Compound 1 (L1): 3-(2-phenoxyethyl)-1,2,3,4-tetrahydrobenzo[4,5] Imidazo[1,2-a][1,3,5] Triazine
1. Introduction
- The benzimidazoles contain a phenyl ring fused to an imidazole ring. Benzimidazole and their derivatives have diverse applications in coordination chemistry, photophysics, photochemistry and bioinorganic chemistry. [1-4] Three component condensation reaction of Benzimidazole is very important for the synthesis of various useful compounds.[5] Over the past few decades, Mannich base reactions of benzimidazole have been the guiding tent for the synthetic chemists because of their widespread pharmaceutical importance i.e. antibacterial [6], anthelmintic[7], antifungal[8], anti-inflamatory[9], antiviral [10] and analgesic[11] properties. In addition to their biological importance, benzimidazoles form stable complexes with various transition metals.[12] Transition metal complexes of 2-substituted benzimidazole and benzimidazole-based mixed ligands have been reported with mono-, bi- and tri- dentate coordination behavior.[13-17] Continuous increase in bacterial resistance to existing drugs has been resulted due to wide spread use of antibacterial agents leading to research on new substances possessing antimicrobial activity.[18,19] Several benzimidazoles are commercially available as pharmaceuticals, veterinary products and fungicides.The worthwhile biological activities of Mannich bases have been guiding for the synthesis of novel Mannich bases. The main objective of present communication is to provide a comprehensive account of N-Mannich type bases of benzimidazole, their chelating behavior and to highlight their potential in evolving better antimicrobial drugs. A total of 3 Mannich bases and 12 metal(II) complexes have been prepared in this study and well characterized by their physical, spectral and analytical data. The synthesized compounds were further evaluated for their antimicrobial properties against various pathogens using MIC method.
2. Experimental Work
2.1. General Manipulations
- All the reagents and solvents were purchased from Sigma-Aldrich and they were used as received. Reactions were monitored by thin layer chromatography (plates coated with 0.2 mm Merck 60 F254 silica gel) and were visualized by UV irradiation (254 nm). Elemental analyses were carried out with a LECO-CHNS-9320 model. 1H-NMR spectra of compounds were recorded with a Bruker Spectrospin Avance DPX-300 using TMS as internal standard and d6 DMSO as solvent. Infrared spectra of compounds were recorded on a Philips Analytical PU 9800 FTIR spectrophotometer. The melting points of compounds were determined with a Gallenkamp melting point apparatus. UV/visible absorption spectra were recorded using a Shimadzu UV-1700 spectrophotometer at room temperature. Conductance was recorded by pre-calibrated cyber scan 500 conductivity meter. Electron impact mass spectra (EIMS) were recorded on a JEOL MS Route instrument. Thermogravimetric analysis (TGA) was carried out under constant nitrogen flow at a heating rate of 15°C min-1, using a Mettler Toledo TGA/SDTA 851 balance. The heating scans were performed on 3-5 mg of sample, in the temperature range 25-900°C. In vitro antibacterial, antifungal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
2.2. Synthesis of Mannich Bases (Scheme 1)
- To a solution of 2-aminobenzimidazole (0.05 mole) in 30 ml of 1-4dioxane, 0.1 mole formaldehyde and 0.05 mole ethanolic solution of respective primary amine were added. The mixture was stir for 2 h at 75°C. A clear solution was obtained. The completion of reaction was monitored by TLC. The obtained solution was filtered and reduced to half of its volume by evaporation of the solvent in vacuo. The concentrated solution was left overnight at room temperature, which led to the formation of a solid product. This solution was filtered, washed with dioxane then with ether and, dried.
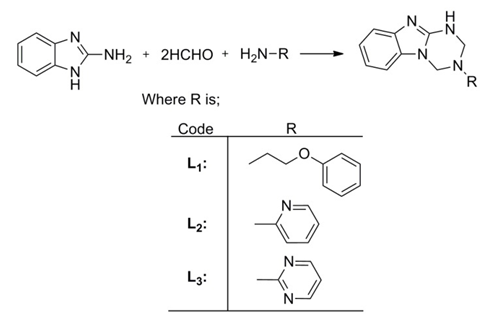 | Scheme 1. Synthesis of Mannich bases |
2.3. Synthesis of Metal Complexes (Scheme 2)
- To a hot magnetically stirred methanolic solution of Mannich bases (L1-L3) (0.1 mole), a methanolic solution of metal(II) salts (0.05 mole) was added. The mixture was then refluxed for 2 h. A clear solution was obtained. The completion of reaction was monitored by TLC. The solution obtained was cooled at room temperature, precipitates appeared were filtered and washed with acetone and dried.
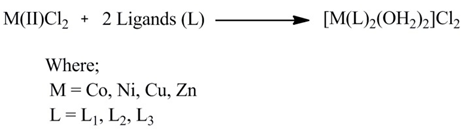 | Scheme 2. Synthesis of metal complexes |
 | Figure 1. Representative IR spectra of L1 |
 | Figure 2. Representative 1NMR Spectra of L1 |
2.4. Compound 1 (L1): 3-(2-phenoxyethyl)-1,2,3,4-tetrahydrobenzo[4,5] Imidazo[1,2-a][1,3,5] Triazine
- White solid; yield 56%; m.p: 175°C; IR (KBr): 3382 (NH stretching), 3055 (Aromatic CH2), 2847 (CH2 Stretching), 1655 (C=N), 1355 (C-N, Amine), 1242 (C-O); 1H-NMR (DMSO-d6): δ 7.2-7.3 (t, 2H, benzimidazole ring), 7.1-7.2 (t, 2H, benzimidazole ring), 6.8-6.9 (m, 5H, phenyl ring), 5.1 (s, 2H, N-CH2-N), 4.3 (s, 2H, N-CH2-NH), 4.1 (t, 2H, -O-CH2), 3.6 (s, 1H, NH), 2.9 (t, 2H, N-CH2-C); Mass spectrum (ESI) [M]+ = 294; Anal. Calcd. for C17H18N4O (294.35) (%): C, 69.37; H, 6.16; N, 19.03. Found (%): C, 69.30; H, 6.12; N, 19.08. 1H-NMR of Zn(II) complex (DMSO-d6): δ 7.4-7.5 (t, 2H, benzimidazole ring), 7.3-7.4 (t, 2H, benzimidazole ring), 7.1-7.3 (m, 5H, phenyl ring), 5.3 (s, 2H, N-CH2-N), 4.5 (s, 2H, N-CH2-NH), 4.3 (t, 2H, -O-CH2), 3.8 (s, 1H, NH), 3.1 (t, 2H, N-CH2-C).
2.5. Compound 2 (L2): 3-(pyridin-2-yl)-1,2,3,4-tetrahydrobenzo[4,5] Imidazo[1,2-a][1,3,5] Triazine
- White solid; yield 42%; m.p: 193°C; IR (KBr): 3382 (NH stretching), 3055 (Aromatic CH2), 2854 (CH2 Stretching), 1660 (C=N), 1574 (NH bending), 1348 (C-N, aromatic amine); 1H-NMR (DMSO-d6): δ 8.0 (d, 1H, pyridine ring), 7.3-7.4 (m, 2H, benzimidazole ring), 7.1 (d, 1H, pyridine ring), 6.9 (m, 2H, benzimidazole ring), 6.8 (s, 2H, N-CH2-N), 6.5-6.6 (m, 2H, pyridine ring), 5.4 (d, 2H, N-CH2-NH), 3.6 (s, 1H, NH); Mass spectrum (ESI) [M]+ = 251; Anal. Calcd. for C14H13N5 (251.12) (%): C, 66.92; H, 5.21; N, 27.87. Found (%): C, 66.87; H, 5.32; N, 27.92. 1H-NMR of Zn(II) complex (DMSO-d6): δ 8.2 (d, 1H, pyridine ring), 7.5-7.6 (m, 2H, benzimidazole ring), 7.4 (d, 1H, pyridine ring), 7.1 (m, 2H, benzimidazole ring), 7.0 (s, 2H, N-CH2-N), 6.7-6.8 (m, 2H, pyridine ring), 5.6 (d, 2H, N-CH2-NH), 3.8 (s, 1H, NH).
2.6. Compound 3 (L3): 3-(pyrimidin-2-yl)-1,2,3,4-tetrahydrobenzo[4,5] Imidazo[1,2-a][1,3,5] Triazine
- White solid; yield 44%; m.p: 207 °C; IR (KBr): 3396 (NH stretching), 3055 (Aromatic CH2), 2867 (CH2 stretching), 1653 (C=N), 1590 (NH bending), 1348s (C-N); 1H-NMR (DMSO-d6): δ 8.6 (t, 1H, pyrimidine ring), 7.5-7.6 (d, 2H, pyrimidine ring), 7.2-7.3 (t, 2H, benzimidazole ring), 7.1 (d, 2H, benzimidazole ring), 6.8 (d, 2H, N-CH2-NH), 6.6 (s, 2H, N-CH2-N), 3.5 (s, 1H, NH); Mass spectrum (ESI) [M]+ = 252; Anal. Calcd. for C13H12N6 (252.27) (%): C, 61.89; H, 4.79; N, 33.31. Found (%): C, 61.82; H, 4.74; N, 33.36. 1H-NMR of Zn(II) complex (DMSO-d6): δ 8.9 (t, 1H, pyrimidine ring), 7.7-7.8 (d, 2H, pyrimidine ring), 7.5-7.6 (t, 2H, benzimidazole ring), 7.3 (d, 2H, benzimidazole ring), 7.0 (d, 2H, N-CH2-NH), 6.8 (s, 2H, N-CH2-N), 3.7 (s, 1H, NH).
2.7. Antibacterial Activity
- The in-vitro antibacterial activity of Mannich bases (L1-L3) and their metal(II) complexes (C1-C12) was assayed against two Gram-negative (Escherichia coli, Pseudomonas aeruginosa) and two Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains by the reported method. [20,21] The stock solution (1 mg/ml) of the test chemical was prepared by dissolving 10 mg of the test compound in 10 ml of Dimethyl sulfoxide (DMSO) solvent. The stock solution was suitably diluted with sterilized distilled water to get dilution of 100, 50 and 25 mgml-1. Control for each dilution was prepared by diluting 10 ml of solvent instead of stock solution with sterilized distilled water. The wells (6 mm in diameter) were dug in the agar media with the help of a sterile metallic borer. Two to eight hours old bacterial inocula containing approximately 104-106 colony forming units (CFU/mL) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The prepared concentrations of the test sample were introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, Gentamycin, served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 24 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). In order to clarify any effect of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains.
 | Figure 3. Representative IR spectra of L2 |
 | Figure 4. Representative 1NMR spectra of L2 |
 | Figure 5. Representative IR spectra of L3 |
 | Figure 6. Representative 1NMR spectra of L3 |
2.8. Antifungal Activity
- All the compounds (L1-L3) and their metal(II) complexes (C1-C12) were studied against five fungal cultures (Aspergillus niger, Penicillium expansum, Rhizopus nigricans, Trichoderna lignorum, Botrydepladia thiobromine) for Antifungal activities. Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 cfu ml-1 fungal spore suspensions and transferred to petri plates. The stock solution of test chemical was prepared and diluted to 100, 50 and 25mgml-1. Discs soaked in 20 ml of prepared concentrations of all compounds were placed at different positions on the agar surface. The plates were incubated at 32 °C. The percentage inhibition was calculated after seven days and compared with standard drugs Fluconazole.[22]
2.9. In vitro Cytotoxicity
- The synthesized compounds and their Zn(II), Co(II), Cu(II) and Ni(II) complexes were screened for their cytotoxicity (brine shrimp bioassay) by using the protocol of Meyer et al.[21] Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 х 32 cm) filled with artificial seawater, which was prepared with a commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the minor compartment was open to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 ml of DMSO. From this stock solution 100, 50 and 25 mgml-1 were transferred to nine vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 ml of DMSO only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 ml of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 ml per vial. After 24 h the number of survivors was counted. Data were analyzed by a Finney computer program to determine the LD50 values.[23]
3. Results and Discussion
3.1. IR Spectra
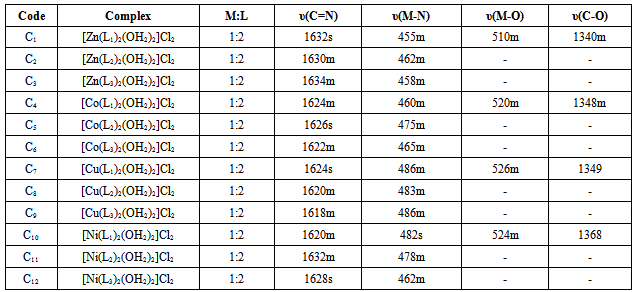
- The important IR spectral bands of the Mannich bases and their metal complexes along with their tentative assignments are given in the experimental and Table 1.The ligands show a broad band at 3382-3396 cm-1 and sharp bands at 1653-1660 cm-1, 2847-2867 cm-1, assigned to NH stretching, υ(C=N) and CH2 stretching vibrations respectively. In the complexes of Ligands 1 and 2, the azomethine frequency shows a downfall (15-30 cm-1) indicating coordination through N atom. This is further supported by the appearance of new bands at 450-486 cm-1 due to υ(M-N) bond.[24]The C-O stretching mode in Ligand 1 is usually found around 1242cm-1. The shift of C-O stretching towards higher frequencies in the metal complexes suggest M-O bond formation and appearance of new bands at 510-540 cm-1 support the formation of M-O bond.[25]The presence of coordinated water molecule in the complex is indicated by the appearance of a broad band at 3226-3460 cm-1 and two weak bands in the region 754-784 cm-1 and 700-718 cm-1 due to (-OH) rocking and wagging mode of vibrations, respectively.[26]
3.2. 1H NMR Spectra
- 1H NMR spectra of the free ligands and their diamagnetic zinc(II) complexes were recorded in DMSO-d6. The 1H NMR spectral data along with the possible assignments is recorded in the Experimental. Mannich bases (L1-L3) have shown the peak at 4.3-6.8 ppm due to methylene linkage (2H, -CH2-) formed between benzimidazole moiety and amino compound. Mannich bases reaction can be further confirmed by the absence of peak for (-NH) secondary amino group of benzimidazole ring system.[27] Two triplet peaks at 4.1 and 2.9 ppm indicated the presence of CH2 groups in L1. A multiplet peak at 6.8-6.9 ppm indicated the presence of phenyl group. In L2, peaks at 8.0, 7.1 and 6.5-6.6 ppm indicated the presence of pyridine ring. Multiplet peaks at 7.3-7.4 and 6.9 ppm confirms the presence of benzimidazole moiety. A triplet peak at 8.6 ppm and doublet peak at 7.5-7.6 ppm indicated the presence of pyrimidine ring in L3. In L3, presence of benzimidazole group indicated by the appearance of triplet peak at 7.2-7.3 and doublet peak at 7.1 ppm. The 1H NMR spectra of Zn(II) complexes lend further support to the mode of bonding discussed in their IR spectra. The coordination of the nitrogen and oxygen is inferred by the downfield shift (0.3-0.6) of the surrounding proton signals in the complexes. All other protons underwent a downfield shift by 0.2-0.4 ppm due to the increased conjugation[28] and coordination with the metal atom.
3.3. Electronic Spectra and Magnetic Moments
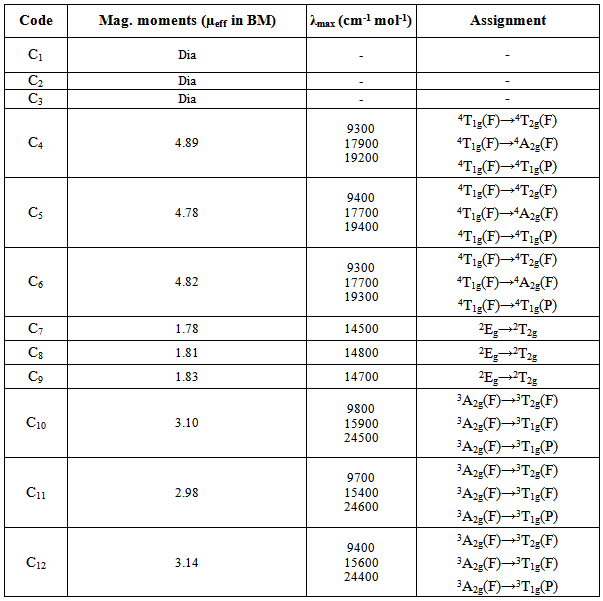
- The electronic spectra if Ni(II) complexes exhibits three bands at 9400-9800, 15400-15900 and 24200-24600, which may reasonably be assignable to 3A2g(F)→3T2g(F), 3A2g(F)→3T1g(F) and 3A2g(F)→3T1g(P) transitions, respectively. The magnetic moments for Ni(II) complexes (2.98-3.27 BM) are within the range of an octahedral geometry.[7] The electronic spectra of Co(II) complexes shows absorption bands at 9200-9400, 17700-17900 and 19200-19500 assignable to 4T1g(F)→4T2g(F), 4T1g(F)→4A2g(F) and 4T1g(F)→4T1g(P) transitions, respectively. The magnetic moment values of Co(II) complexes are 4.78-4.89 BM, suggesting an octahedral geometry.[29] The observed magnetic moments for Cu(II) complexes are 1.72-1.83 BM and the band observed at 14500-14900 (2Eg→2T2g) in the electronic spectra suggest an octahedral geometry.[30] The Zn(II) complexes are diamagnetic as expected for d10 system. (Table 2)
|
|
|
3.4. Thermogravimetric Analysis
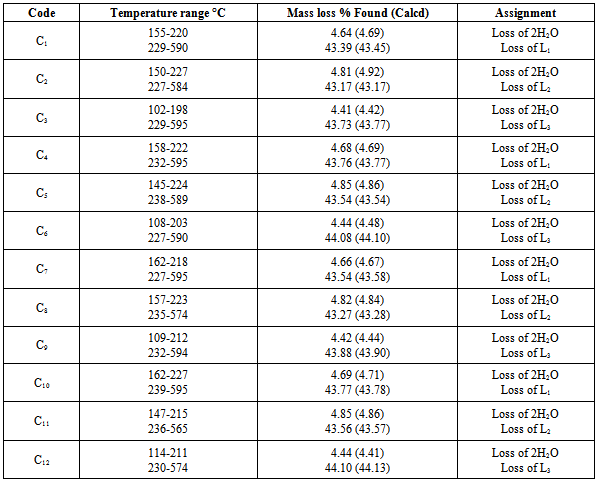
- Thermogravimetric analyses (TGA) for the complexes were carried out from room temperature to 700°C. Coordinated waters are usually eliminated at higher temperatures than those of hydration[31,32] usually in the temperature range 100-350°C. The complexes may decompose in more than two steps with the formation of intermediates[33,34] calculated and estimated mass losses are comparable. The TGA curves of all Mannich-base metal complexes (C1-C12) have two stages of mass loss, at 102-227°C and at 227-595°C. Weight loss in the range 102-227°C with estimated mass loss of 4.1-4.85% in all the complexes indicates the loss of two coordinated waters. From 227°C to 595°C, a sharp decrease in weight indicated the loss of one Mannich base from the complexes with estimated mass loss of 43.17-44.10% for all the complexes respectively(Table 4).The molecular masses determined mass spectrometrically (Table 3) also confirmed the ML2 composition. Based upon experimental evidence thus obtained, the complexes were characterized as six coordinates with the two positions occupied by water. The hydrated complexes have significant importance in the enzymatic systems, as the substrates can bind to metal by substituting the coordinated water. The proposed structures of the complexes under investigation, on the basis of above experimental evidence, are shown in Figure 7, 8 and 9. Unsuccessful attempts to isolate crystals suitable for X-ray analysis prevented further structure elucidation.
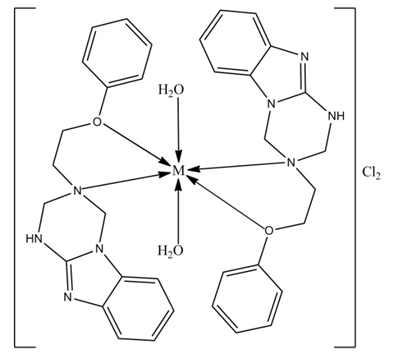 | Figure 7. Proposed structure of metal complexes of L1, where; M=Co(II), Ni(II), Cu(II) and Zn(II) |
 | Figure 8. Proposed structure of metal complexes of L2, where; M=Co(II), Ni(II), Cu(II) and Zn(II) |
|
3.5. Antibacterial Activity
- The in vitro antibacterial activity was assayed against two Gram-positive (Bacillus subtilis Staphylococcus aureus) and two Gram-negative strains (Escherichia coli, Pseudomonas aeruginosa) according to the reported method.[35] Gentamycine was used as a comparative drug. (Figure 10).
3.6. Antifungal Activity
- Antifungal activity was determined in vitro against Aspergillus niger, Penicillium expansum, Rhizopus nigricans, Trichoderna lignorum and Botrydepladia thiobromine. The inhibition results were compared with the standard drug Fluconazole. (Figure 11)Mannich bases expressed lower antifungal activity as compared to antibacterial. All the derivatives were efficacious against A. niger and P. expansum. The results for R. nigricans and T. lignorum were satisfactory only by a high concentration, showing zone of inhibition in 60-70% range. B. thiobromine was almost insusceptible for all the Mannich bases, but showed moderate results for complexes at higher concentration. (Table 6)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.7. Cytotoxic Bioassay
|
 | Figure 10. In vitro antibacterial spectrum of Mannich bases (L1-L3) and Zn(II), Co(II), Cu(II) and Ni(II) complexes (C1-C12) and gentamycine (Std.) at 100 µgml-1 concentration |
 | Figure 11. In vitro antifungal spectrum of Mannich bases (L1-L3) and Zn(II), Co(II), Cu(II) and Ni(II) complexes (C1-C12) and Fluconazole (Std.) at 100 µgml-1 concentration |
4. Conclusions
- The synthesized Mannich bases act as bidentate ligands. The IR, TGA, conductivity, magnetic and electronic studies confirms that the ligands coordinated to metal through oxygen and nitrogen as donor atoms. All the derivatives and their metal(II) complexes were evaluated in vitro against four bacterial (two Gram-negative, two Gram-positive) and five fungal strains. Compounds showed more potency against bacteria. E. coli, P. aeruginosa, A. niger and P. expansum were the most susceptible species.
ACKNOWLEDGEMENTS
- The author thanks to the Higher Education Commission (HEC), Government of Pakistan for awarding Indigenous Scholarship and supporting research facilities. We also thank to QAU, Islamabad for providing spectroscopic services. Finally, HEJ Research Institute of Chemistry, University of Karachi is also acknowledged for undertaking the biological assays.
References
| [1] | X.C. Huang, J.P. Zhang, X.M. Chen, A New Route to Supramolecular Isomers via Molecular Templating: Nanosized Molecular Polygons of Copper (I) 2-Methylimidazolates, J. Am. Chem. Soc., 126 (2004) 13218–13219. |
| [2] | T.J. Cardwell, A.J. Edwards, R.M. Hartshorn, R.J. Holmes, Structural and Electrochemical Studies of Some Cobalt (III) Complexes of 2-Aminomethylbenzimidazole and 2-(N-Methylaminomethyl) benzimidazole, Aust. J. Chem., 50 (1997) 1009–1016. |
| [3] | Y.P. Tong, S.L. Zheng, Synthesis, structure, spectroscopic properties, DFT and TDDFT investigations of copper(II) complex with 2-(2-hydroxyphenyl) benzimidazole, J. Mol. Struct., 841 (2007) 34–40. |
| [4] | Y.P. Tong, S.L. Zheng, X.M. Chen, Syntheses, structures, photoluminescence and theoretical studies of two dimeric Zn (II) compounds with aromatic N,O-chelate phenolic ligands, J. Mol. Struct., 826 (2007) 104–112. |
| [5] | K.S. Shikhaliev, A.Y. Potapov, D.V. Krylskii, 2-Aminobenzimidazole in three-component cyclization reactions with formaldehyde and primary amines, Russian Chemical Bultn. Int. Ed., 56 (2007) 367–368. |
| [6] | Misbah ur Rehman, M. Imran, M. Arif, M. Farooq. Metal- based Antimicrobial Agents: Synthesis, Characterization and Biological Studies of Mannich Base Derivatives of Benzimidazole and Their Metal Complexes. Science Journal of Chemistry, 1[5] (2013) 56–66. |
| [7] | S. Murugesan, S. Sathiyamoorthy, Synthesis, Spectral analysis and Biological activity of Mannich Bases of 2-Substituted benzimidazole, J. Pharm. Res., 4 (2011) 2679–2681. |
| [8] | V. Kamlesh V, Patel, S. Arun, Synthesis, Characterization and Chelating Properties of Benzimidazole-Salicylic Acid Combined Molecule, Eur. J. Chem., 6 (2009) 281–288. |
| [9] | B.A. Reddy, Synthesis, Characterization and biological evaluation of 1,2-disubstituted benzimidazole derivatives using Mannich bases, Eur. J. Chem., 7 (2010) 222–226. |
| [10] | S. Periyasamy, R.L. Dhani, P. Christophe, Synthesis, anti-viral and cytotoxicity studies of some novel n-substituted benzimidazole derivatives. Int. J. Pharmaceut. Sci. Res., 1 (2010) 105–109. |
| [11] | E.P. Jesudason, S.K. Sridhar, E.J. Padma, et al., Synthesis, pharmacological screening, quantum chemical and in vitro permeability studies of N-Mannich bases of benzimidazoles through bovine cornea, Eur. J. Med. Chem., 44 (2009) 2307–2312. |
| [12] | H. Marijana, S. Kristina, K. Sandra, et al., Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles, Eur. J. Med. Chem., 46 (2011) 2274–2279. |
| [13] | K.C. Rout, R.R. Mohanty, S. Jena, K.C. Dash, Dioxouranium (VI) and thorium (IV) complexes with 2(2′-pyridyl) 1-methylbenzimidazole and reaction of dioxouranium (VI) complex with mercury (II), cobalt (II) and nickel(II), Polyhedron, 15 (1996) 1023–1029. |
| [14] | H. Chang, M. Fu, X.J. Zhao, E.C. Yang, Four benzimidazole-based ZnII/CdII polymers extended by aromatic polycarboxylate coligands: synthesis, structure, and luminescence, J. Coord. Chem., 63 (2010) 3551–3564. |
| [15] | F.M.Nie, J. Chen, Z. Li, F. Lu, Synthesis and characterization of picolinate-containing nickel (II) complexes with tridentate and tripodal tetradentate ligands, J. Coord. Chem., 63 (2010) 1711–1719. |
| [16] | M.Y. Duan, J. Li, Y. Xi, X.F. Lu, J.Z. Liu, G. Mele, F.X. Zhang, Synthesis and characterization of binuclear manganese (IV, IV) and mononuclear cobalt (II) complexes based on 2-(2-hydroxyphenyl)-1H-benzimidazole, J. Coord. Chem., 63 (2010) 90–98. |
| [17] | M. Akkurt, S. Karaca, H. Kucukbay, E. Orhan, O. Buyukgungor,Dichlorobis[1-(2-ethoxyethyl)-1H-benzimidazole- N3] nickel (II), Acta Cryst., 61(E) (2005) m41–m43. |
| [18] | M. Arif, M.M.R. Qurashi, M.A. Shad, Metal-based antibacterial agents: synthesis, characterization, and in vitro biological evaluation of cefixime-derived Schiff bases and their complexes with Zn(II), Cu(II), Ni(II), and Co(II), J. Coord. Chem., 64 (2011) 1914–1930. |
| [19] | H. Kucukbay, S. Gunal, E. Orhan, R. Durmaz, Synthesis and Antimicrobial Activities of Some Transition Metal Benzimidazole Complexes, Asian J. Chem., 22 (2010) 7376–7382. |
| [20] | R.M. Mannar, K. Amit, E. Martin, R. Dieter Synthesis, characterization, reactivity, and catalytic potential of model vanadium (IV, V) complexes with benzimidazole-derived ONN donor ligands, Inorg. Chem., 45 (2006) 5924–5937. |
| [21] | Atta-ur-Rahman, M.I. Choudhary, W.J. Thomsen WJ, Bioassay techniques for drug development, Harwood Academic Publishers, The Netherlands (2001). |
| [22] | K.S. Anil, M. Yasmin, R.A. Kamal, P. Om, Hypervalent iodine mediated synthesis of1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents, Eur. J. Med. Chem., 38 (2003) 533–536. |
| [23] | B.N. Meyer, N.R. Ferrigni, J.E. Putnam, et al., Brine shrimp: a convenient general bioassay for active plant constituents, Planta Med., 45(1982) 31–34. |
| [24] | A.W. Bauer, W.M. Kirby, J.C. Sherris, M. Turck, Antibiotic susceptibility testing by a standardized single disk method, Am. J. Clin. Pathol., 45 (1966) 493–496. |
| [25] | K. Nakamoto, Infrared and raman spectra of inorganic and coordination compounds, 5th edn. John Wiley & Sons Inc. New York (1997). |
| [26] | J.R. Ferrero, Low-frequency Vibrations of Inorganic and Coordination Compounds, Wiley-Interscience, New York (1971). |
| [27] | R. Sayaji, Synthesis, Magneto-Spectral and Biological Studies of Manganese (II) Complexes with Heteroaroyl Hydrazones, Asian J. Chem., 17 (2005) 2663–2668. |
| [28] | W.W. Simons, The Sadtler handbook of proton NMR spectra, Sadtler Research Laboratories Inc. Philadelphia (1978). |
| [29] | R.K. Agarwal, P. Garg, H. Agarwal, S. Chandra, Synthesis, Magneto-Spectral and Thermal Studies of Cobalt (II) and Nickel(II) Complexes of 4-[N-(4-Dimethylaminobenzylidene) amino] Antipyrine, Synth. React. Inorg. Met.-Org. Chem., 27 (1997) 251–268. |
| [30] | P.K. Panchal, M.N. Patel, Synthesis, Structural Characterization, and Antibacterial Studies of Some Mixed‐Ligand First Row d‐Transition Metal Complexes, Synth. React. Inorg. Met.-Org. Chem., 34 (2004) 1277–1289. |
| [31] | H. Koksal, M. Dolaz, M. Tilmer, S. Serin, Copper(II), cobalt(III), nickel(II), palladium(II), and zinc(II) complexes of the Schiff base ligands derived from 2,6-diacetylpyridine and phthaldialdehyde, Synth. React. Inorg. Met.-Org. Chem., 31 (2001) 1141–1162. |
| [32] | G.G. Mohamed, Z.H. Abd El-Wahab, Salisaldehyde- 2-aminobenzimidazole schiff base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II), J. Therm. Anal. Calorim., 73 (2003) 347–359. |
| [33] | Y.M. Essa, H.M. Abdel-Fattah A.A. Soliman, Thermogravimetric and spectroscopic studies on La(III) and Ce(III) complexes with some thio-schiff bases, J. Therm. Anal., 12 (1994) 1175–1184. |
| [34] | N.T. Abdel-Ghani, O. Esherif, Potentiometric,conductimetric, spectrometric, thermogravimetric and magnetic studies of lanthanum complexes with some symmetric 1,5-diaryl-3- cyanoformazans, Thermochim. Acta, 156 (1989) 69–83. |
| [35] | M.A. Zayed, F.A. Nour El-Dien, G.G. Mohamed, M.E.A. El-Gamel, Structure investigation, spectral, thermal, X-ray and mass characterization of piroxicam and its metal complexes, Spectrochim. Acta, 60 (2004) 2843–2852. |
| [36] | Z.H. Chohan, C.T. Supuran, A. Scozzafava, Isatin-derived antibacterial and antifungal compounds and their transition metal complexes. J. Enzyme Inhib. Med. Chem., 19 (2004) 417–423. |
| [37] | Z.H. Chohan, M. Praveen, Synthesis, characterization, coordination and antibacterial properties of novel asymmetric 1,1′-disubstituted ferrocene-derived Schiff-base ligands and their Co(II), Cu(II) Ni(II) and Zn(II) complexes, Appl. Organomet. Chem., 15 (2001) 617–625. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML