-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2013; 3(5): 140-147
doi:10.5923/j.chemistry.20130305.04
Fe3O4 Modification of Microcrystalline Cellulose for Composite Materials
Kiril V. Dimitrov1, Michael Herzog2, Sanchi Nenkova1
1University of Chemical Technology and Metallurgy, Sofia, 1756, Bulgaria
2Technische Hochschule - Wildau, Wildau, 15745, Germany
Correspondence to: Kiril V. Dimitrov, University of Chemical Technology and Metallurgy, Sofia, 1756, Bulgaria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A new synthesis method for producing cellulose ferrite micro- and nano- composites was developed and new material properties were studied. Microcrystalline cellulose was modified with a mixture of Fe+2/Fe+3 to produce surface bonded nanoparticles magnetite (Fe3O4). Optimal conditions were determined. Microsized hematite (Fe2O3) was mixed with microcrystalline cellulose and used as a reference. The magnetite modified microcrystalline cellulose and hematite filled microcrystalline cellulose were used together with polyurethane prepolymer. New composite crosslinked conductivity materials based on the magnetite modified microcrystalline or hematite filled microcrystalline cellulose and polyurethane were developed. Morphology, crystalline properties, water absorption and electro conductivity of these materials were characterized. The physical properties of these materials were characterized by different analytical methods: SEM, XRD, water absorption and electrical resistance.
Keywords: Microcrystalline cellulose, Magnetite, SEM, XRD
Cite this paper: Kiril V. Dimitrov, Michael Herzog, Sanchi Nenkova, Fe3O4 Modification of Microcrystalline Cellulose for Composite Materials, American Journal of Chemistry, Vol. 3 No. 5, 2013, pp. 140-147. doi: 10.5923/j.chemistry.20130305.04.
Article Outline
1. Introduction
- Large amounts of magnetic particles have been produced for magnetic recording media in the past fifty years, while recently, magnetic particles have also been extensively used in a variety of applications such as ferrofluids, magnetic inks, for means of extraction and purification of biological materials and clinical diagnostics[1]. Magnetite (Fe3O4) particles represent a very interesting type of magnetic materials, being used among others in information storage, magnetic fluids and medical applications (i.e. drug carriers), as well as in materials for the absorption of electromagnetic radiation. Such a wide variety of possible applications has warranted a lot of attention and has resulted in a large body of research work concerning the synthesis of magnetite[2,3].Composite materials are of interest due to the potential synergistic properties that may arise from the combination of two or more components. Two such components are wood or cellulose fibers (length 20 mm) and magnetic nanoparticles. In combination organic (fiber) and inorganic (particles) compounds forming hybrid materials possessing the inherent properties of the fiber substrate, in particular stiffness and strength, and also the magnetic properties of thenanoparticles. Superparamagnetic iron oxide nanoparticles (SPION) covered with a polymer have been used in medical research such as devices for cell isolation, immobilization of enzymes, controlled release systems and separation of biological materials[4]. Guo et al. reported the study of inorganic adsorbents inside cellulose beads for the elimination of arsenic from aqueous solutions[5]. Thus, cellulose beads could act as a column bed, suitable for liquidchromatography. Another way of using cellulose beads is to place magnetic particles inside the structure and thus the new material could react as a magnetic actuator. Moreover, the synthesis of the magnetic cellulose[6] or polysaccharide like carboxymethyl-cellulose[7] and carrageenan[8] hasgenerated a great attention in recent years due to their specific properties: non-toxic nature, biocompatibility, variable ionic permeability, combined with a high hydrophilic effect and considerable mechanical strength. The immobilization of α-amylase onto cellulose-coated magnetite nanoparticles has been reported in a starch degradation study[9]. Previous work describes the synthesis of cellulose beads bearing micrometric magnetite[10]. In this initial report, the covered micrometric magnetite in a cellulosic matrix leads to a net reducing effect of magnetic coercive field with consequential formation of superparamagnetic cellulose composite. Magnetically responsive cellulose fibers will allow the investigation of new concepts in papermaking and packaging, security paper, and information storage. Potential applications are in electromagnetic shielding, magneto graphic printing and magnetic filtering[6, 11-12]. Throughout the literature, there have been reports of super paramagnetic papers obtained through “in situ” synthesis of ferrites[11–13] and others concerning ferromagnetic paper obtained by the “lumen-loading” approach, whereby the lumen of the cellulose fiber is loaded with commercial pigments such as magnetite and maghemite.The purpose of this work is to modify microcrystalline cellulose (MCC) with two component system Fe+2/Fe+3, in order to obtain composite materials with desired properties, based on such modified MCC (mMCC) and polyurethane (PU) prepolymer. PU prepolymer served as a polymer component with active –NCO groups, which reacted with the active –OH groups from MCC.
2. Materials
- For the synthesis of the composite matrix, following reagents were used:MCC with particle size 5 μm - supplied from Sigma Aldich Chemie GmbH, Steinheim Germany. Modifying agents used were: FeCl2∙4H2O – supplied from Мalilinckrodt Baker B.V, Deventer Germany, M=198.8 g/mol and FeCl3∙6H2O – supplied from Merck KGaA, Darmstadt Germany, M=270.3 g/mol. Hematite (Fe2O3) with particle size 5 μm was used as a referent sample, delivered from Sigma Aldrich Chemie GmbH, Steinheim Germany. 30 w% NH3 solution – supplied from Carl Roth GmbH & Co.KG, Karlsruhe Germany, M=17.03 g/mol, density 0.9g/cm3.1–isocyanato-4 dicyclohexylmethan-4,4’-diisocyanate (H12-MDI) under the trade name Vestanat, supplied from Performance Chemicals Handels GmbH, Hamburg, Germany, M=262 g/mol, and Lupranol 3300, which is a trifunctional polyether polyol based on glycerol, were used for a prepolymer preparation. Lupranol 3300 had only secondary hydroxyl group (hydroxyl value = 400 mg KOH/g), and was supplied from BASF Polyurethanes GmbH, M=420 g/mol.
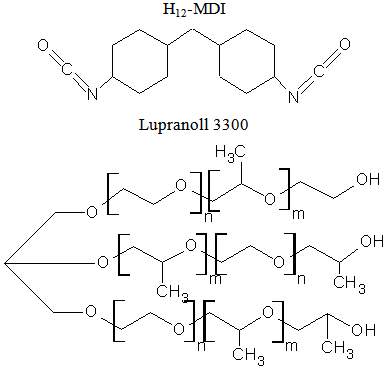 m, n = 1- 10
m, n = 1- 103. Experimental Section
3.1. MCC Modification
- The MCC (600 g) was in bulk treated with 1 mol solution (300 ml) of FeCl2∙4H2O and 2 mol solution (300ml) of FeCl3∙6H2O and a suspension was produced. The reconstituted material was left for 30 minutes to absorb ferrite solutions. After 30 minutes this suspension was treated with 30 w % NH3 solution and was vigorously stirred for 20 min. The reconstituted material was filtrated and washed with distillate watter. The finished product should have a neutral pH. Such modified MCC was dried for 3 hours at 105℃. Fe3O4 was produced by the following reaction[14]:
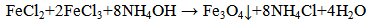 | (1) |
3.2. Preparation of Polyurethane Prepolymer
- Polyurethane (PU) prepolymer was synthesized using H12-MDI and Lupranol 3300. Procedure:H12-MDI was fed to a vessel and heated up to 50 – 60℃. Fresh H12-MDI gives a clear colourless liquid; a slight turbidity because of beginning dimerization of H12-MDI may be accepted. Lupranol 3300 was added in small portions at room temperature, stirring during the course of approximately 30 minutes. The reaction is exothermic, but the reactivity of Lupranol 3300 is very low, therefore crystallization of the H12-MDI may occur in cases when the content of the vessel cools down under 40℃. The mixture is heated up to 80℃ and kept at this temperature for 3 hours. After 3 hours, the NCO-content should be stable. The result is a clear, slightly yellow, viscous prepolymer. At the end of the experiments, quality control tests were performed to determine NCO-content of the product according to DIN EN ISO 11 909. Compositions and synthesis conditions are listed in Table 1.
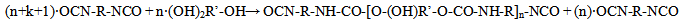 | (2) |
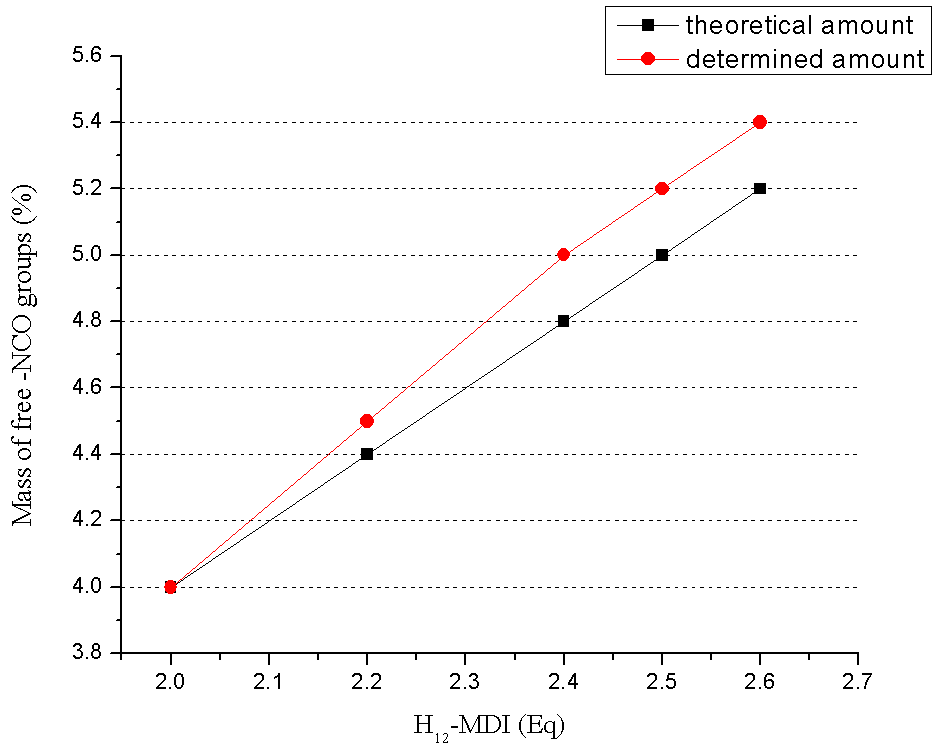 | Figure 1. NCO content of PU prepolymer |
3.3. Preparation of Composite Materials
- In present work, the K5 prepolymer was used to synthesize all samples described in Table 2. The composite synthesis is based on the physical mixing and chemical bonding of MCC and PU prepolymer. Mixing was carried out in Brabender-Plasti® Corder Lab Station, equipped with Mixer 350 E. Calculated amounts (200 g) of both the components were added to the apparatus and mixed for 15 min at 180o C with rotation speeds of 20 min-1. Then the material was pressed at 4 MPa in the laboratory press Servitec/Polystat 300 S. Table 2 represents the contents of the composite materials.
|
 | (3) |
4. Analysis Methods
4.1. Scanning Electron Microscopy (SEM)
- For the SEM investigation, specimens with surface areas of about 5x5 mm and thickness about 0,1 mm were taken from modified MCC. The samples were coated with gold by argon sputtering. Subsequently surface morphology of the materials was investigated by SEM - JSM∙6400 (voltage = 20.0 kV and magnification 500-17000).
4.2. X-ray Diffraction (XRD)
- XRD investigation of composites was performed using D-8Advance from Bruker AXS, equipped with a Ge(111)- Johansson monochromator Cuka (λ=1 540598 Ǻ). The measurements were performed in the range of scattering angles 2 θ from 10o to 80o, with a step width of 0.01o. XRD was used for structural phase identification Figure 3, 4. Coherently diffracting domain size (dxrd) was calculated from the width of the XRD peak under the Scherrer approximation (which assumes the small crystallite size to be the cause of line broadening) after correcting for instrumental broadening.
4.3. Water Absorption
- Water absorption of composites was used to determine the amount of water absorbed under specified conditions. This research was done according DIN 53 496 The measurement was done at samples with a length of 2 cm, width of 1 cm and thickness from 2.4 mm to 2.9 mm were immersed in a glass of water for 24, 48 and 72 hours at ≈ 25℃.
4.4. Electrical Resistance
- This measurement was done using Agilent N9320B RF Spectrum Analyzer, with scanning frequency in the range 1 MHz to 3 GHz. The samples thickness ranged from 2,4 mm to 2,9 mm. The steep of frequency was 20 Hz. The test was performed at room temperature ≈ 25℃.
5. Results and Discussion
- The prepared composite materials were characterized by SEM, XRD, water absorption, mechanical properties and electro conductivity.Figure 2. (A, B, C) represents SEM micrographs of Fe3O4 mMMC powder scanned with different resolutions. The micrographs show MCC particles covered with magnetite with different size (nano- and micro- size), thus some of the particles agglomerate during the modification process.
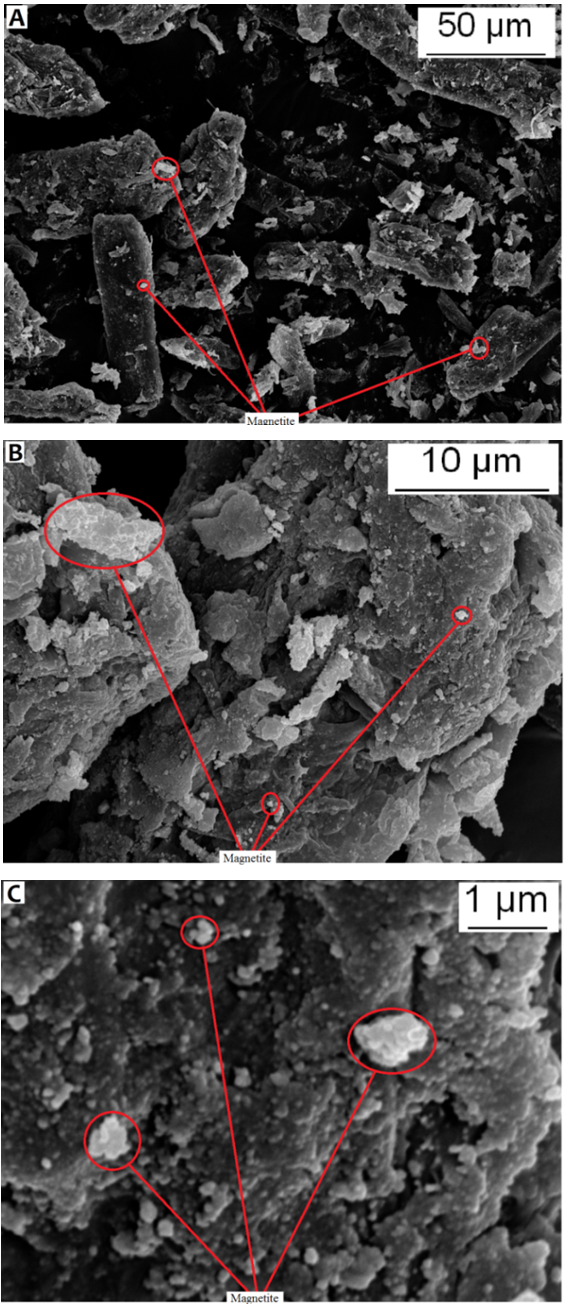 | Figure 2. SEM of F3O4 mMCC |
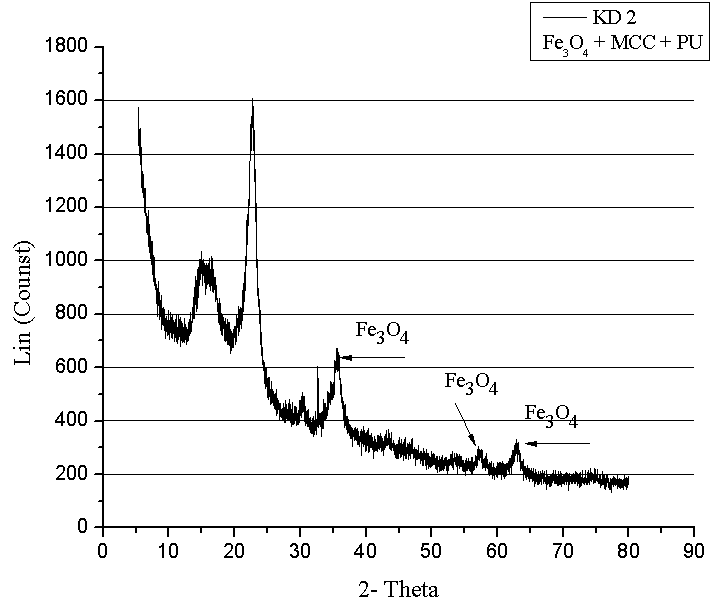 | Figure 3. X-ray structure of composite materials with Fe3O4 mMCC |
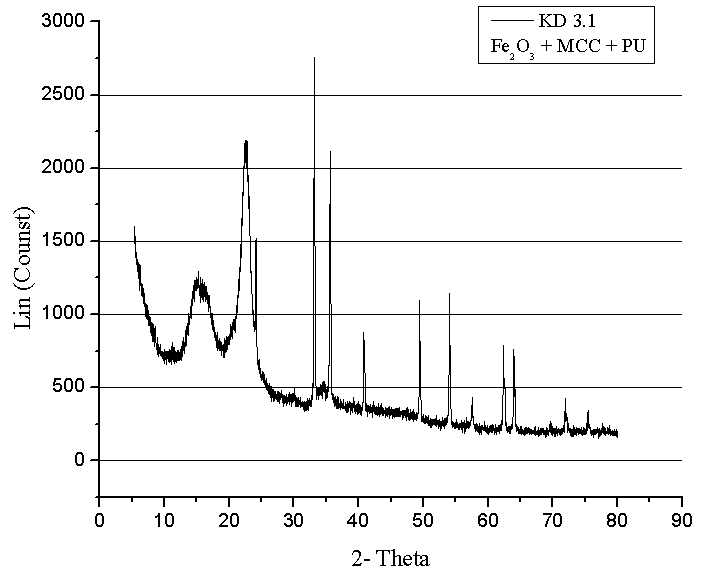 | Figure 4. X-ray structure analysis of composite materials with MCC and hematite |
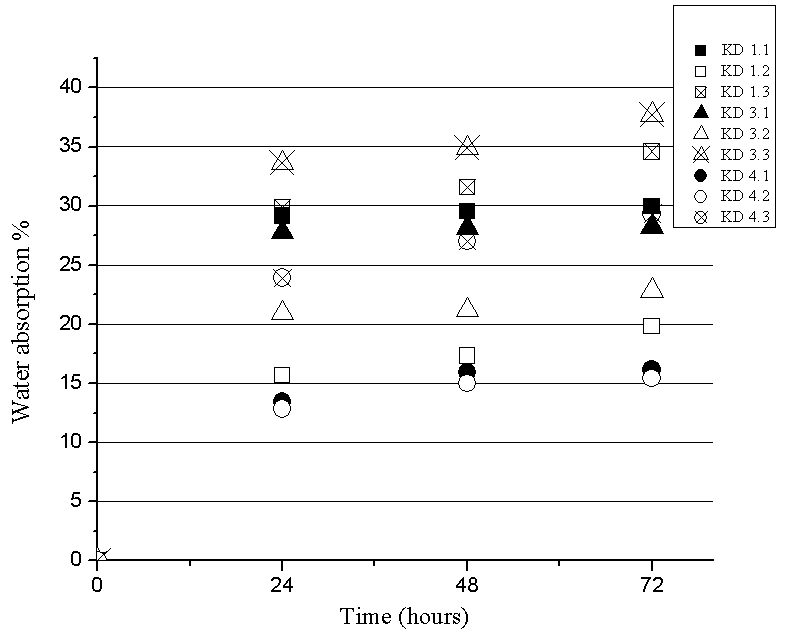 | Figure 5. Dependence on water absorption of time |
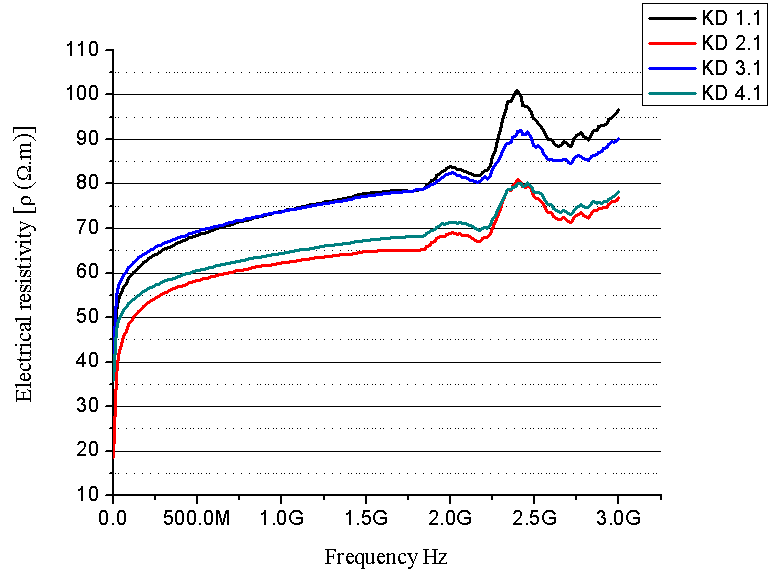 | Figure 6. Electrical resistivity as a function of frequency (composites with 30 % PU prepolymer) |
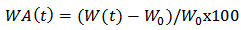 | (4) |
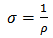 | (5) |
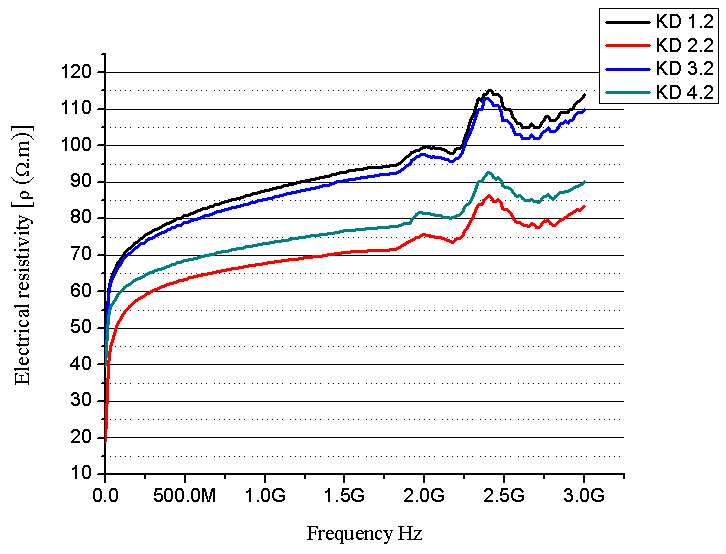 | Figure 7. Electrical resistivity as a function of frequency (composites with 20 % PU prepolymer) |
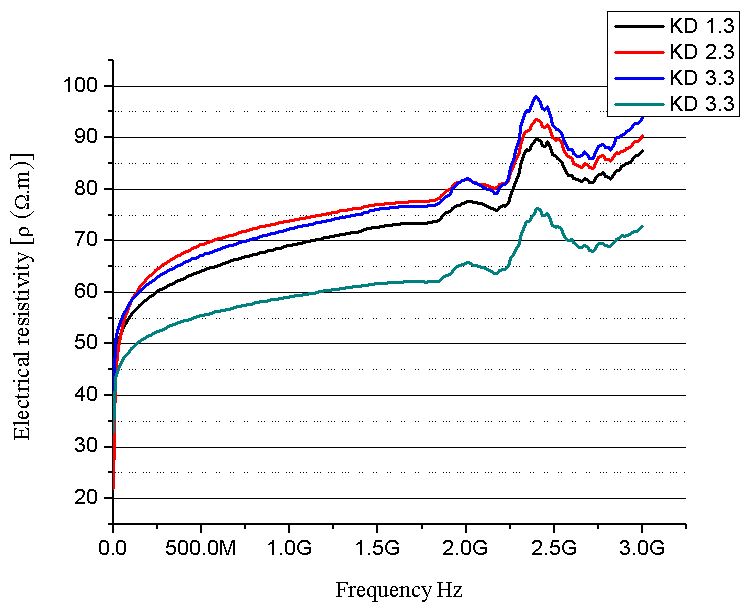 | Figure 8. Electrical resistivity as a function of frequency (composites with 10 % PU prepolymer) |
6. Conclusions
- SEM and XRD investigation show successful MCC – magnetite modification by magnetite. Both investigations show the presence of nano-sized magnetite. As can be seen from SEM micrographs, the micro-sized magnetite particles coexist with nano-sized ones. This fact could be explained by the agglomeration process during the modification of MCC. XRD investigation confirms that the particles are magnetite.The water absorption degree is an indication that the hematite-filled cellulose composites are more suitable for use in water environment compared to the Fe3O4 mMCC composite materials.Electrical resistivity measurements show that the samples with Fe3O4 mMCC have higher values of electrical conductivity than the composite with pure MCC. This can be an indication for successful modification of MCC also. These kinds of materials are possible to have electromagnetic absorption properties and can be used for electromagnetic shielding.The whole investigation shows that modification of MCC with two-component system of Fe+2 and Fe+3 followed by its chemical transformation in nano- and micro-sized magnetite particles is possible and provides the possibility for further investigation in this direction.
References
| [1] | M. Ozaki, E. Matijevic and M. Borkovec. “Magnetic particles: preparation, properties, and application” in: (Eds.),Surface and Colloid Science, vol. 17, Academic/Plenum Publishers, New York, 2004, pp. 1–26. |
| [2] | Y. Lee, J. Lee, C. J. Bae, J.-G. Park,H.-J. Noh, J.-H. Park, T. Hyeon “Large-Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions” vol. 15, Advanced Functional Materials (2005), pp. 503-509. |
| [3] | S. Sun and H. Zeng “Size-Controlled Synthesis of Magnetite Nanoparticles” Journal American Chemical Society, 124 (28), 2002, , pp 8204–8205. |
| [4] | J. E. Ramírez-Vick, A. A. García and J. Lee, “Immobilization of silver ions onto paramagnetic particles for binding and release of a biotin-labeled oligonucleotide” vol. 43, Reactive and Functional Polymers, (2000) pp. 53-62. |
| [5] | X. Guo, Y. Du, F. Chen, H.S. Park, Y. J. Xie, “Mechanism of removal of arsenic by bead cellulose loaded with iron oxyhydroxide (beta-FeOOH)” EXAFS study.Colloid Interface Sci. 314 (2007) pp. 427-33. |
| [6] | A. C. Small, and J. H. Johnston, “Novel hybrid materials of magnetic nanoparticles and cellulose fibers” vol. 331, J. Colloids Interf. Sci., (2009), pp. 122–126. |
| [7] | P. Sipos, “Manufacturing of size controlled magnetite nanoparticles potentially suitable for the preparation of aqueous magnetic fluids” vol. 58, Roman. Rep. Phys. (2006) 269. |
| [8] | A. L. Daniel-da-Silva, T. Trindade, B. J. Goodfellow, B. F. Costa, R. N. Correia, A. M. Gil, “In situ synthesis of magnetite nanoparticles in carrageenan gelsBiomacromolecules” vol. 8, J. Biomacromolecules, (2007), pp. 2350-2357. |
| [9] | M. Namdeo and S. K. Bajpai, “Immobilization of α-amylase onto cellulose-coated magnetite (CCM) nanoparticles and preliminary starch degradation study” vol. 59, J. Mol. Catal. B: Enzym. (2009), pp. 134-139. |
| [10] | J. R. Correa, D. Canetti, E. Bordallo, J. Rieumont, J. Dufour, “Application of cubic magnetite to the synthesis of super paramagnetic cellulose beads for enzyme immobilization” Proceedings of the 8th Inter American Congress of Electron Microscopy, Inter American Committee of Societies of Electron Microscopy, Rev. Biotecnol. Aplic., La Habana, Cuba, September (2005), 25–30. |
| [11] | R. H. Marchessault, P. Rioux, L. Raymond, “Magnetic cellulose fibres and paper: preparation, processing and properties” vol. 33, Polymer Journal, (1992), pp. 4024-4028. |
| [12] | L. Raymond, J.-F. Revol, D. H. Ryan and R. H. Marchessault, “In situ synthesis of ferrites in cellulosics” vol. 6, Chem. Mater., (1994), pp. 249–255. |
| [13] | J. A. Carrazana-Garcia, M. A. Lopez-Quintela, J. Rivas-Rey, “Characterization of ferrite particles in presence of cellulose fibers” vol. A 121, Colloids Surf. (1997) pp. 61-66. |
| [14] | H. D. Hunger, K. Vaskova, Präparation undCharakterisierung von biologisch aktiven Magnetit-Protein-Nanopartikeln “ TFH Wildau, Wissenschaftliche Beiträge (2006), pp. 47-50. |
| [15] | M. Ma, Y. Zhang, W. Yu, H. Y. Shen, H. Q. Zhang, N. Gu, Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf A: Physicochem Eng Aspects 2003; 212:219–226. |
| [16] | S. Chen, J. Feng, X, Guo, J. Hong, W. Ding, One-step wet chemistry for preparation of magnetite nanorods. Mater Lett 2005;59: 985–998. |
| [17] | W. Voit, D. K. Kim, W. Zapka, M. Muhammed, K. V. Rao. Magnetic behavior of coated superparamagnetic iron oxide nanoparticles in ferrofluids. Mater Res Soc 2001;676: 781–786 |
| [18] | S. Nenkova, P. Velev, M. Dragnevska, D. Nikolova, and K. Dimitrov, Conductive composite with CuS. BioResources 6(3), 2356-2365 (2011) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML