-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2013; 3(5): 126-135
doi:10.5923/j.chemistry.20130305.02
Thiophene-Based Azo Dyes and Their Applications in Dyes Chemistry
Moaz M. Abdou
Egyptian Petroleum Research Institute, Nasr city, P.O. 11727, Cairo, Egypt
Correspondence to: Moaz M. Abdou, Egyptian Petroleum Research Institute, Nasr city, P.O. 11727, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The thiophene-based azo dyes class of azo dyes have generated considerable interest since its discovery 62 years ago. These dyes have come to be applied to a very large number and variety of fibres and have therefore achieved an importance which is probably greater than the weights used would suggest. The connection between dyeing and fastness properties on the one hand and chemical constitution on the other is in general indefinite. Also, some factors affecting the current application of these dye types are discussed.
Keywords: Thiophene, Azo Dyes, Dyeing Properties, Fastness Properties, Chemical Constitution
Cite this paper: Moaz M. Abdou, Thiophene-Based Azo Dyes and Their Applications in Dyes Chemistry, American Journal of Chemistry, Vol. 3 No. 5, 2013, pp. 126-135. doi: 10.5923/j.chemistry.20130305.02.
Article Outline
1. Introduction
- Azo dyes are a versatile class of coloured organic dyes and receive a large amount of attention in the literature, as a consequence of their exciting biological properties and their applications in various fields, such as textiles, papers, leathers, additives, cosmetics and organic synthesis[1–5]. Interest in the design of azo dyes containing heterocyclic moieties stems from their high degree of brightness shade compared to analogous dyes derived from carbocyclic aromatic systems[6-8]. Additionally, this class of dyes was established as an alternative to more expensive anthraquinone dyes for both environmental and economic reasons[9,10]. In recent years, the use of heterocyclic intermediates in the synthesis of azo disperse dyes is well established, and the resultant dyes exhibit good tinctorial strength and excellent brightness[11,12]. More recently, dyes prepared from various heterocyclic anilines including 2-aminothiophenes have been evaluated as disperse dyes. A typical example in this respect are those containing azothiophene and its benzo analogues moieties that deserve special attention due to their color deepening effect and small molecular structure leading to better dye ability and intrinsic conjugation. The heterocyclic nature of the thiophene ring has also allowed for excellent sublimation fastness on the dyed fibers[13,14]. Moreover, the sulfur atom plays a decisive role by acting as an efficient electron sink as explained by valence band theory[15]. It has now been conclusively established that ring systems of this type are useful for providing red-violet and greenish-blue colors that meet the rigorous technical and economic requirements demanded of them by both manufacturer and user[16-21].Before the 1949s, almost all of the dyes available were prepared from either substituted anilines or substituted anthraquinones. The prolific development of the colors of monoazo dyes containing thiophene rings actually emanated from 1950[22]. While, the first investigations into monoazo dyes based on 5-acetyl-2-amino-3-nitrothiophene by Eastman Kodak[24] in the 1950s showed that these dyes had rather high bathochromism and performance. A systematic study by Dickey[23] was published which established the better relative bathochromy of thiophene azo dyes over their carbocyclic counterparts and provided qualitative evidence of the higher absorption intensities of the former dye type.A one-pot procedure by Gewald[25-27] to the synthesis of 2-aminothiophenes sparked the renewed commercial interest in these compounds as diazo components. The promise of Gewald's discovery signaled a burst of patent activity[28], which was followed by a steady stream of applications concerning thiophene-based azo disperse dyes over the next 30 years[10]. A number of researchers studied aminothiophene derivatives as azo disperse dyes in the dyeing of synthetic fibers[29] and blended polyester/wool fibers[30,31].Despite 62 years having elapsed since thienyl-2-azo dyes were first scrutinized systematically, and the continued industrial interest in them, prior to the end of the previous decade, only a very limited amount of information pertaining to the synthesis, properties and application of such dyes existed outside the patent literature. Since then, however, papers have appeared dealing with each or all of these aspects. While focusing on the multifunctional activities of such compounds, this review briefly points out to the current trends in dye design and development of newer dyeing molecules, which hold future promises in dyes chemistry.
2. Modifications to the Thiophene Nucleus
- A new key finding in the evolution of azothiophenes was modification of the thiophene nucleus through the addition of different substituents at the different positions. These modifications altered the dyeing and fastness properties of the thiophenes and provided a better understanding of the structure–activity relationship (SAR) in azothiophene compounds. The addition of specifically selected substituents at these key positions on the thiophene nucleus made it possible to target specific groups of dye and to improve the dyeing properties of the earlier quinolone compounds. A wider variety of 2-aminothiophenes, prepared directly by the Gewald synthesis or after subsequent dramatization, [32] were used to produce a range of yellow-red to green dyes.[33] Raising the total electron withdrawing strength of the substituents in the thiophene ring not only led to the need for increasingly acidic diazotizing media through weakening of the basicity of the amino group, but also resulted in bathochromism shifts (in agreement with theory and the Pariser–Parr–Pople molecular orbital (PPPMO)calculations), hyperchromism, increased positive solvatochromism and a reduction in the size of observed positive halochromic shifts.[34] The presence of two strong acceptors or a nitro group on the heterocyclic ring was sufficient to bring about negative halochromism.
2.1. 2-aryl/hetarylazothiophene
- The most interesting advance in this field has been the emergence of 2-aminothiophenes as diazo components. Disperse dyes based on suitably di-(ortri-)substituted-2-aminothiophenes were comprehensively patented in 1972 primarily by ICI,35a and also by Kodak, 35b Sandoz 35c and Gewald.35d During the last 35-40 years, developments in the synthetic routes to substituted 2-aminothiophenes have resulted in considerable interest in these amines as diazo components for blue dyes for polyester, cellulose acetate and polyamide fibres.36-38 The investigated and developed diazo components include 3.5-dinitro,39,40 3-cyano-5- nitro,41,42 3-cyano-4-methyl-5-nitro,41,43 3-ethoxycarbonyl-5- nitrothiophene,44 4-methyl-5-cyanothiophene,453,5-dicyano4-methylthiophene, 46 3-carboalkoxy-5-nitro,413-carboalkoxy-4-methyl-3-nitro-, 41,42 5-cyano-4-methyl-3- nitro-,47 3-alkylsulphonyl-5-nitro-,41 3-carboxy-5-nitro-,48 and 3-formyl-5-nitrothiophene.49 Moreover, blue acid dyes 50,51 for nylon carpet fibre have been patented from certain selected 2-aminothiophene diazo components, whose future development will be watched with interest.
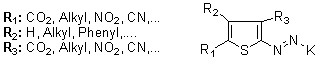 | Figure 1. Chromophore and structures of the main 2-aryl/hetaryl azothiophenes that have been approved for dyeing use |
2.1.1. 2-Aryl Azothiophenes
2.1.1.1. Nitro-substituted
- A series of greenish blue monoazo dyes was prepared by treating diazotized 5-substituted 2-amino-3-nitrothiophens with aniline coupling components, and still greener dyes than that prepared with tetrahydroquinoline and benzomorpholine couplers. It is of considerable significance, both theoretically and technically, that mononitro-substituted of 2-aminothiophens give blue dyes even with simple aniline couplers. The benzene analogues of these dyes are orange to red. In the phenyl azo dye series a diazo component containing two nitro groups and a third negative group, such as chloro or ethylsulphamoyl, is required to give even a violet dye with the same coupler.
2.1.1.1.1. 3,5-Dinitrothiophene-2-azodyes
- Azo dyes based on 3,5-dinitrothiophene, as the most important class of this type to date, are represented by the two ICI-patented 3,5-dinitrothiophene derivatives Blue XVI 52 and Blue XIX.53 Also, green, navy and black colorants formulated from them are marketed worldwide, the first of which was CI Disperse Green 9, derived from 2-amino-3,5-dinitrothiophene. These dyes also have the valuable property of flaring green when exposed to artificial light, thus rendering them valuable as shading components. Other colorants based on this diazo component find use in alkali dischargeable/clearable formulations,54,55 as stand alone blues and, more importantly, in navy or black mixtures.
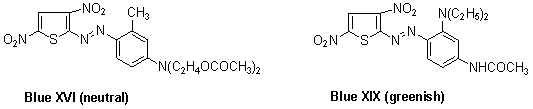 In 2004, Kyriaki and coworkers showed that bluish-red azo dyes 1 were prepared from 2-amino-3,5-dinitrothiophene were diazotized and coupled to substituted N-β-acetoxyethylanilines. These dyes had moderate to high uptake on cellulose acetate, excellent wash fastness, and moderate to high light fastness depending on the substituents in the diazo and coupling component and the depth of dyeing.56
In 2004, Kyriaki and coworkers showed that bluish-red azo dyes 1 were prepared from 2-amino-3,5-dinitrothiophene were diazotized and coupled to substituted N-β-acetoxyethylanilines. These dyes had moderate to high uptake on cellulose acetate, excellent wash fastness, and moderate to high light fastness depending on the substituents in the diazo and coupling component and the depth of dyeing.56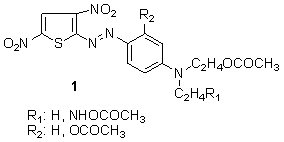 Azo disperse dyes containingN-2-hydroxyethyl-1-naphthylamine 2 can be produced via a Bucherer reaction and then used as a coupler to make blue azo dye when coupled amino thiophene. The resultant dye obtained give moderate colouration of cellulose acetate, good wash fastness, and moderate light fastness.57
Azo disperse dyes containingN-2-hydroxyethyl-1-naphthylamine 2 can be produced via a Bucherer reaction and then used as a coupler to make blue azo dye when coupled amino thiophene. The resultant dye obtained give moderate colouration of cellulose acetate, good wash fastness, and moderate light fastness.57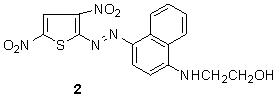
2.1.1.1.2. 5-Substituted-3-nitrothiophene-2-azodyes
- The 3,5-dinitro-2-thienyl azo dyes are, in general, not as lightfast on polyesters as are dyes from 2-amino-5-acyl-3-nitrothiophenes, 23, 58and patents have claimed dyes from this latter diazo component which have outstanding light fastness and sublimation fastness on polyesters.59-61 Although blue 3-nitro-5-acetylthiophene2-azodyes were already described in 1958,23 this type was again taken up from 1979 onwards 62 and expanded to include fluorinated 5-acyl,63 5-methylsulphonyl, 64 5-(substituted)benzoyl 65 and finally 5-cinnamoyl groups.66,67 Dyes from 2-amino-3-nitro-5-trifluoroacetylthiophene 68 are also valuable for preparing heat transfer printing dyes.Other negatively substituted thiophene dyes developed in early work were derived from 2-amino-3-nitro-5-alkylsulphonylthiophenes and 2-amino-3,5-dialkylsulphonylthiophenes,69 however, these dyes were hypsochromic in shade to the dyes from 2-amino-3-nitro-5-acylthiophenes. 70
2.1.1.1.3. 3-Substituted-5-nitrothiophene-2-azodyes
- The extremely greenish blue of Blue XIX has so far not been attained by the AQ dye Blue VI or the azo benzene derivatives such as Blue XVIll or other heterocyclicazodyes. This was undoubtedly the motive for renewed patent activities mainly on the part of Japanese dye manufacturers in the area of 3-cyano5-nitrothiophene2-azodyes. 71, 72
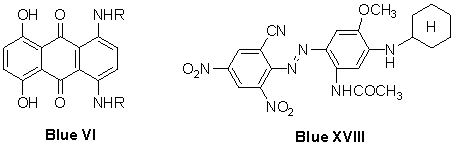 Dyes derived from 2-amino-3-cyano-5-nitrothiophene have been extensively patented in the past, and have featured in recent patents concerning the dyeing and printing of hydrophobic fabrics, 71, 72, 73,74 for example, in the preparation of blue colorants which can be applied under alkaline conditions, 75 a dye of this kind has been commercialized and introduced to the Japanese market. 76 Specific crystal modifications of particular structures derived from this diazo component, as well as post-synthetic methods for obtaining them, have been claimed. 77-80 Patents covering the use of this class of dyes in mixtures formulated with aminoazobenzene- and anthraquinone-based colorants have also appeared, for example, in the production of blue, 81-84 as well as green, turquoise and navy dyestuffs. 85Alaa et al. 86 reported that azothiophene dyes 4 are prepared via diazotizing of substituted 2-aminothiophenes 3 using nitrosyl sulfuric acid with appropriate couplers, such as 2,3-dihydroxynaphthalene, resorcinol, 2-(N-methylanilino)ethanol, 2-(N-ethylanilino)ethanol, 3-[(2-hydroxyethyl)phenylamino]propionitrile (Scheme 1).
Dyes derived from 2-amino-3-cyano-5-nitrothiophene have been extensively patented in the past, and have featured in recent patents concerning the dyeing and printing of hydrophobic fabrics, 71, 72, 73,74 for example, in the preparation of blue colorants which can be applied under alkaline conditions, 75 a dye of this kind has been commercialized and introduced to the Japanese market. 76 Specific crystal modifications of particular structures derived from this diazo component, as well as post-synthetic methods for obtaining them, have been claimed. 77-80 Patents covering the use of this class of dyes in mixtures formulated with aminoazobenzene- and anthraquinone-based colorants have also appeared, for example, in the production of blue, 81-84 as well as green, turquoise and navy dyestuffs. 85Alaa et al. 86 reported that azothiophene dyes 4 are prepared via diazotizing of substituted 2-aminothiophenes 3 using nitrosyl sulfuric acid with appropriate couplers, such as 2,3-dihydroxynaphthalene, resorcinol, 2-(N-methylanilino)ethanol, 2-(N-ethylanilino)ethanol, 3-[(2-hydroxyethyl)phenylamino]propionitrile (Scheme 1). 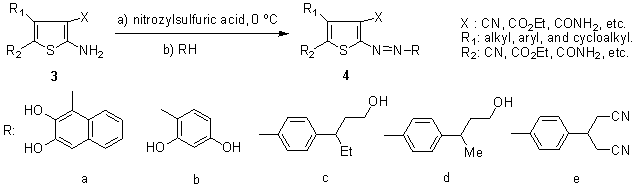 | Scheme 1. Synthesis of azothiophene dyes 4 |
2.1.1.2. 3-Carbethoxythiophene-2-azodyes
- 2-Amino-3-carbethoxy-5-nitrothiophene, a precursor for the dye 5, can be prepared by reaction of 1,4-dithiane with ethyl cyanoacetate and subsequent nitration.87
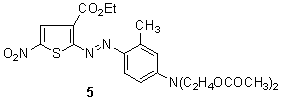 Maradiya 88-93synthesized a series of disperse polymeric dyes by free-radical polymerization of monomeric dyes that were synthesized by diazotation of 2-aminothiophene derivatives, 2-amino-3-carbethoxy-4,5-dimethylthiophene or 2-amino-3,5-bis-(ethoxycarbonyl)-4-methylthiophene, and coupling with various N-arylmaleimides. These dyes were applied as a disperse dyes on nylon and polyester fibres. They were found to give various color shades with good to very good depth and levelness on the fiber. The variation in the shades of dyed fibres are due to the nature and position of the various substituent present on the maleimide and thiophene ring. The dyeing of the monomeric dyes showed good fastness to light and very good to excellent fastness to washing, perspiration, sublimation, and solvents. The percentage dye bath exhaustion on nylon fabric has been found to be good and acceptable. Also, the corresponding polymeric dyes showed excellent fastness properties. Thus, the improvement of the fastness properties with increasing the molecular size of the dye molecule by polymerization reaction leads to brilliancy of shade and excellent fastness properties.
Maradiya 88-93synthesized a series of disperse polymeric dyes by free-radical polymerization of monomeric dyes that were synthesized by diazotation of 2-aminothiophene derivatives, 2-amino-3-carbethoxy-4,5-dimethylthiophene or 2-amino-3,5-bis-(ethoxycarbonyl)-4-methylthiophene, and coupling with various N-arylmaleimides. These dyes were applied as a disperse dyes on nylon and polyester fibres. They were found to give various color shades with good to very good depth and levelness on the fiber. The variation in the shades of dyed fibres are due to the nature and position of the various substituent present on the maleimide and thiophene ring. The dyeing of the monomeric dyes showed good fastness to light and very good to excellent fastness to washing, perspiration, sublimation, and solvents. The percentage dye bath exhaustion on nylon fabric has been found to be good and acceptable. Also, the corresponding polymeric dyes showed excellent fastness properties. Thus, the improvement of the fastness properties with increasing the molecular size of the dye molecule by polymerization reaction leads to brilliancy of shade and excellent fastness properties.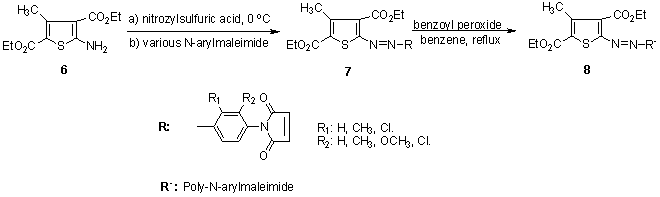 | Scheme 2. Synthesis of polymeric dyes 8 by free-radical polymerization of monomeric dyes |
2.1.1.3. 5-Formyl thiophene-2-azodyes
- 5-Formylthiophene2-azo dyes with hydrogen, alkyl or aryl in the 4-position were patented by various authors.94-102 It was noted that the condensation products were claimed,103 in which the aldehyde group was modified analogously to Blue XI. Also, the patent application described the diazotization of 3-negatively substituted-2-amino5-formylthiophenes coupled to 2-N,N-dialkylamino-4-arylthiazoles.104 As, 4-chloro5-formyl(cyano)thiophene2-azodyes including derivatives of the aldehyde as blue chromophores have been described.105a The substituent in the 3-position of the 5-formylthiophenediazo components is preferably cyano, such as 8.105b.
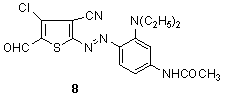
2.1.1.4. N-Alkylaminothiophene-2-azodyes
- 2-N,N-dialkylaminothiophenes with various substituents in the 3-position and occasionally alkyl or phenyl groups in the 4-position, coupled with isocyclic as well as heterocyclic diazo components, are claimed in Japanese patent applications.106 These compounds were already disclosed by BASF in 1977 as terminal coupling components of bisazo dyes.107 In 1999, a series of thienyl azo disperse dyes 9 has been prepared from their corresponding coupling components, 2-aminothiophenes. Depending on the various substituents present in the diazo component, absorption maxima varied from 437 to 534 nm in toluene. 108
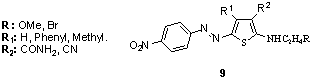 An important commercial aspect of certain dyes derived from 2-aminothiophenes is their sensitivity towards alkali, which can destroy the chromophore, allowing discharging or clearing without the need for reducing agents. 54,55 Not only does this property confer better fastness in tests employing alkaline washing liquors, but it also permits removal of any disperse dye cross-staining the cellulosic component of polyester-cotton/viscose blends with mild alkali, improving wet fastness, brightness and productivity. 169
An important commercial aspect of certain dyes derived from 2-aminothiophenes is their sensitivity towards alkali, which can destroy the chromophore, allowing discharging or clearing without the need for reducing agents. 54,55 Not only does this property confer better fastness in tests employing alkaline washing liquors, but it also permits removal of any disperse dye cross-staining the cellulosic component of polyester-cotton/viscose blends with mild alkali, improving wet fastness, brightness and productivity. 1692.1.1.5. 5-Phenylazothiophene-2-azodyes
- Navy disazo dyes containing thienyl middle components have also been marketed and structures, for example 10 (X = Cl, R1 = CN, R2 = Et), 82,83,109,110 as well as improved methods, 111 have continued to be the subject of patents. The synthesis of disazo disperse dyes whereby2-amino-4,5,6,7-tetrahydrobenzo[b]thiophenes are utilized as the first component with phenyl 112 or naphthyl 113 middle components has been described. Red-brown to blue shades were obtained on polyester, however, light fastness was found to be generally only low.
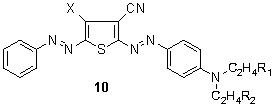 Reddish navy blue of 5-phenylazothiophene-2-azodyes have been intensively patented in Japan 114 since 1980 (approx. 30 applications). This ‘renaissance’ of 5-(substituted)-phenylazothiophene-2-azo dyes 11 and their intermediates is somewhat surprising, considering the thiophene patent literature of the years 1972-75 115-118 and especially in view of the relevant basic dye patent of Bayer. 119
Reddish navy blue of 5-phenylazothiophene-2-azodyes have been intensively patented in Japan 114 since 1980 (approx. 30 applications). This ‘renaissance’ of 5-(substituted)-phenylazothiophene-2-azo dyes 11 and their intermediates is somewhat surprising, considering the thiophene patent literature of the years 1972-75 115-118 and especially in view of the relevant basic dye patent of Bayer. 119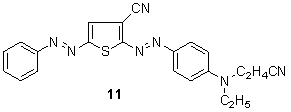
2.1.2. Hetarylazothiophen dyes
- The brilliant red lightfast dyes can be produced by coupling of diazotized thiophen amines to diaminopyridines, 120,121 such as 12, have excited commercial activity. Structures have also been claimed in which aminothiophenes have been coupled to 2,4,6-triamino-3-cyano-pyridines to yield lightfast red colorants for polyester.122
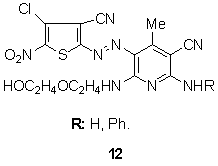 Other heterocyclic couplers explored include 8-aminoquinolines, for example, in the case of blue 13,123 has been disclosed as suitable for the manufacture of dischargeable colorants and lightfast blue dyes, respectively, from 2-aminothiophene-based diazo components.
Other heterocyclic couplers explored include 8-aminoquinolines, for example, in the case of blue 13,123 has been disclosed as suitable for the manufacture of dischargeable colorants and lightfast blue dyes, respectively, from 2-aminothiophene-based diazo components.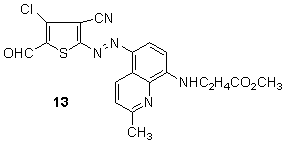 3,5-Substituted-4-methyl-2-aminothiophenes were diazotized and coupled with 4-aryl-2-aminothiophenes to produce red to blue hetarylazo dyes 14. The absorption spectra of these dyes are found to exhibit a strong solvent dependence that varies with the dielectric constants of the solvents. 128
3,5-Substituted-4-methyl-2-aminothiophenes were diazotized and coupled with 4-aryl-2-aminothiophenes to produce red to blue hetarylazo dyes 14. The absorption spectra of these dyes are found to exhibit a strong solvent dependence that varies with the dielectric constants of the solvents. 128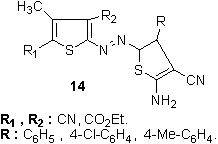 A series of red to blue hetarylazo dyes 15 with good fastness was synthesized by coupling reaction of 4-aryl-2-aminothiophenes derivatives with diazotized 5-nitro-2-aminothiazole and 6-nitro-2-aminobenzothiazole, respectively, as diazo components. The electronic absorption properties of these dyes are found to exhibit a strong solvent dependence that varies with the dielectric constants of the solvents. 129
A series of red to blue hetarylazo dyes 15 with good fastness was synthesized by coupling reaction of 4-aryl-2-aminothiophenes derivatives with diazotized 5-nitro-2-aminothiazole and 6-nitro-2-aminobenzothiazole, respectively, as diazo components. The electronic absorption properties of these dyes are found to exhibit a strong solvent dependence that varies with the dielectric constants of the solvents. 129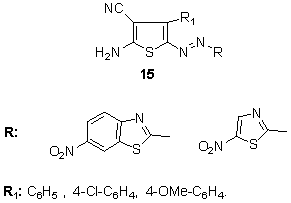 In addition, a series of red to purple or violet bis-hetarylmonoazo dyes derived from different heterocycles 16 has been synthesized from the coupling reactions of diazotized diazo components, poly substituted 2-aminothiophenes with the corresponding coupling components, 2-pyridone and 5-pyrazolone derivatives, respectively. These dyes are found to exhibit a strong solvent dependence which shows a variation with the dielectric constants of the solvent. 130
In addition, a series of red to purple or violet bis-hetarylmonoazo dyes derived from different heterocycles 16 has been synthesized from the coupling reactions of diazotized diazo components, poly substituted 2-aminothiophenes with the corresponding coupling components, 2-pyridone and 5-pyrazolone derivatives, respectively. These dyes are found to exhibit a strong solvent dependence which shows a variation with the dielectric constants of the solvent. 130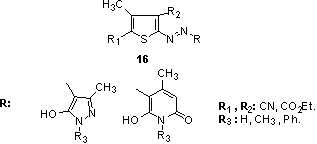 Other derivatives which have been disclosed either for the preparation of disperse dyes, or as colorants in their own right, include related 4-chlorothiophenamines,131 2-aminothiophenes bearing maleimido residues in the 5-position, 132 for example diaminoazothiophene 17.133
Other derivatives which have been disclosed either for the preparation of disperse dyes, or as colorants in their own right, include related 4-chlorothiophenamines,131 2-aminothiophenes bearing maleimido residues in the 5-position, 132 for example diaminoazothiophene 17.133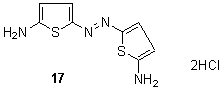 The novel feature of the definition of various heterarylazo groups such as pyridyl-3(benzo)thiazolyl, 1,3,4-thiadiazolyl134 or2-amino-5-(2`-thienylazo)thiophenes diazo components were also published.135A series of yellow to greenish-blue aziridinylazo dyes 19 and their azo precursors containing a thienyl coupling moiety136, 137 has been prepared from2-amino-4-phenylthiophene coupling components using conventional diazo coupling reactions and subsequent cyclisations in good yield. These dyes applied to conventional polyester fibre as well as microdenier polyester by high temperature exhaust dyeing. Heat transferability of these dyes onto polyester fibre has also been examined, using conventional heat-transfer printing techniques. Fabrics dyed with aziridinyl dyes are more resistant to solvent extraction than those dyed with conventional dyes. Residual liquors showed only a pale colour when fabric dyed with aziridinyl dyes was dissolved and then precipitated, whereas a coloured polyester precipitate was obtained.
The novel feature of the definition of various heterarylazo groups such as pyridyl-3(benzo)thiazolyl, 1,3,4-thiadiazolyl134 or2-amino-5-(2`-thienylazo)thiophenes diazo components were also published.135A series of yellow to greenish-blue aziridinylazo dyes 19 and their azo precursors containing a thienyl coupling moiety136, 137 has been prepared from2-amino-4-phenylthiophene coupling components using conventional diazo coupling reactions and subsequent cyclisations in good yield. These dyes applied to conventional polyester fibre as well as microdenier polyester by high temperature exhaust dyeing. Heat transferability of these dyes onto polyester fibre has also been examined, using conventional heat-transfer printing techniques. Fabrics dyed with aziridinyl dyes are more resistant to solvent extraction than those dyed with conventional dyes. Residual liquors showed only a pale colour when fabric dyed with aziridinyl dyes was dissolved and then precipitated, whereas a coloured polyester precipitate was obtained.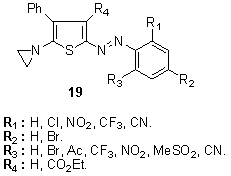
2.2. 4-Aryl/hetarylazothiophen Dyes
- Recently, a set of4-aryl/hetarylazo-3-hydroxy-2-substituted-thiophene disperse dyes 22,23 were synthesized by heterocyclization of ethyl2-arylhydrazono-2-phenylthiocarbamoylacetates 21 with several halocarbonyl reagents, e.g. chloroacetone, phenacyl chloride, and ethyl chloroacetate in the presence of sodium ethoxide (Scheme 3). All of them were investigated for their dyeing characteristics on polyester fabric. The dyed fabrics exhibit very good washing and perspiration fastness properties, with little variations in the moderate to good rubbing fastness. The light fastness is moderate although the incorporation of a nitro group in the diazonium components results in an improvement in light fastness to good.138,139
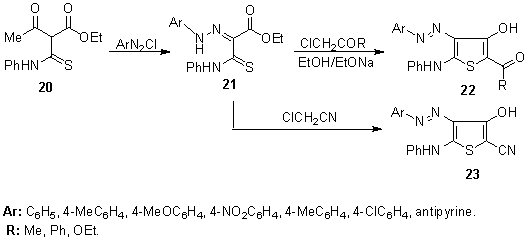 | Scheme 3. Synthesis of 4-aryl/hetarylazo-3-hydroxy-2-substituted-thiophene disperse dyes 22, 23 |
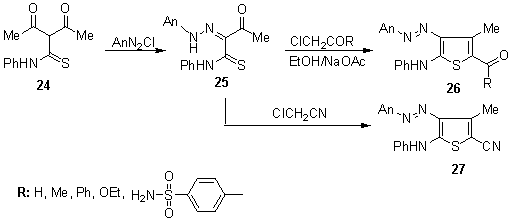 | Scheme 4. Synthesis of functionalized thiophene disperse dyes 26, 27 |
3. Conclusions
- This review has attempted to highlight the key discoveries made during the evolution of the thiophene-based azo compounds class of azodye agents. The currently approved indications for the most commonly used azo thiophenes have also been reviewed, along with the concern of emerging dyeing application to these agents. Clearly, the azo thiophenes have captured the interest of investigators during the past six decades. Finally, it should be noted that this area of chemistry of thiophene-based azo dyes still has much to contribute to organic synthesis and the development of new, important dyes.
ACKNOWLEDGMENTS
- We gratefully acknowledge the researchers who carried out the work described herein. They are individually identified in the references. Finally, Academy of Scientific Research and Technology, ASRT, Egypt, Grant, is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML