-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2013; 3(4): 96-104
doi:10.5923/j.chemistry.20130304.02
Synthesis and Characterization of Highly Effective Nano Sulfated Zirconia over Silica: Core-Shell Catalyst by Ultrasonic Irradiation
S. D. Sharma , Shailandra Singh
Analytical Research Laboratory, School of Sciences, IFTM University, Moradabad, India
Correspondence to: S. D. Sharma , Analytical Research Laboratory, School of Sciences, IFTM University, Moradabad, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A new, simple and convenient sonochemical method was used to synthesize nano sized sulfated zirconia over silica core (NSZS) in which nano units of sulfated zirconia dispersed over silica core were formed in sol-gel medium applying ultrasonic irradiation. Synthesized core-shell catalyst system full fills the requirement of large surface to volume ratio; necessary for highly effective catalyst. The synthesis was carried out in sonochemical glass reactor which consists of ultrasonic horn made up of titanium. By this method, the crystalline size of prepared catalyst was 8 nm. The synthesized catalysts were characterized using XRD, N2 Physiosorption, TG-DTA, FTIR, and SEM. Catalyst shows higher activity for isobutane alkylation with butene-1 at 98% after 26 h of reaction time. After regeneration four times and two times, the activity was over 94% and 92% respectively. These characteristics of NSZS demonstrate that ultrasonic irradiation method allows the synthesis of non-agglomerated nanoparticles fisted onto silica surface.
Keywords: Sulfated Zirconia, Nano Crystalline, Core-Shell, Ultrasonic Irradiation, Silica, Alkylation
Cite this paper: S. D. Sharma , Shailandra Singh , Synthesis and Characterization of Highly Effective Nano Sulfated Zirconia over Silica: Core-Shell Catalyst by Ultrasonic Irradiation, American Journal of Chemistry, Vol. 3 No. 4, 2013, pp. 96-104. doi: 10.5923/j.chemistry.20130304.02.
Article Outline
1. Introduction
- Zirconia has been widely used as catalyst and catalytic support for many chemical reactions [1-5]. Sulfated zirconia shows dual functionalities like beta Zeolite used in acid-promoted catalytic reactions, such as alkylation, isomerization and cracking [6]. Although the zeolite possess larger surface areas, but the deposition of carbon and the formation of coke in and on them limit their industrial applications as catalysts. The small surface area of sulfated zirconia bounds its limits but large surface area always been sought. To increase surface area, various methods such as synthesis of nano crystalline [1-4] and mesoporous [5] Zirconia have been used. Sulfated Zirconia with higher surface area has vast importance in hydrocarbon industry processes such as isomerization, alkylation, etherification, esterification [7-15] and other reactions.There are many ways to synthesise nano sulfated Zirconia that has relatively large surface area, such as reverse micro emulsion, sol-gel technique [16] and simple calcinations without solvent [17]. Sol-Gel technique was reported as one step sol-gel method and two step sol-gel method, while both monoclinic and tetragonal nano sulfated Zirconia with high surface area were prepared by one step sol-gel method[18]. The core –shell system of nano sulfated zirconia over silica support have attracted high attention because of higher surface area and good reactivity [19]. Recently mesoporous silica impregnated with nano crystalline sulfated zirconia was prepared by a sol-gel process using an ionic liquid-template route [20]. It is well-known that many factors can influence acidity of sulfated zirconia catalysts, such as preparation procedure, crystalline phase of zirconia, calcination temperature, sulfur species, surface area, and water content [21-25]. As for preparation procedure, because of different hydrolysis rates of ZrO2 and SiO2 precursors, a phase separation (colloid agglomeration) occurs during the sol–gel reactions. Consequently, after thermal treatment, a Sulfated Zirconia nanocatalyst with a large surface to volume ratio cannot be obtained, although Sulfated Zirconia is able to partly move onto the silica surface during calcination [26]. In addition, the acid strength, as indicated by the concentration of SO42-, is not adjustable by the techniques of co-precipitation and impregnation as reported in the literature [27]. It is apparent that the use of silica carrier as described above could not achieve high catalytic efficiency of sulfated zirconia. An alternative way of synthesis of highly reactive sulfated zirconia over carrier involves the use of ultrasonic irradiation or sonochemical method [28].The application of ultrasonic irradiation to chemical processes dates back to 1930s. However, in the last decade the expansion of the ultrasonic irradiation has become increasingly important [29]. Ultrasonic irradiation provides rather unusual reaction conditions (a short duration of extremely high temperatures and pressures in liquids) that cannot be realized by other methods. Interestingly, such extraordinary conditions are not derived directly from ultrasound itself as no direct molecular level interaction between ultrasound and the chemical species takes place. Instead acoustic cavitation (i.e the formation, growth and implosive collapse of bubbles in liquids) driven by high intensity ultrasound accounts for the chemical effects of ultrasound [30]. When liquids are irradiated with ultrasound, the alternating expansive and compressive acoustic waves create bubbles (i.e., cavities) and make them oscillate. The oscillating bubbles can accumulate ultrasonic energy effectively while growing to a certain specific micrometre size [31]. The shock wave induced by ultrasonic irradiation dislodges the atoms from the surfaces. The dispersed atoms then nucleate and grow into colloidal nano particles, while ultrasound is not directly employed to reduce metal salts in the reaction solution[32]. Ultrasound has often been utilized to deposit nanoparticles onto the surface of substrates. Gedanken and co-workers reported sonochemical deposition of in-situ generated noble metal nanoparticles on various substrates (e.g., silica, carbon, or polymer) [33-35]. Sonochemical deposition by ultrasonic irradiation of inorganic nanoparticles on solid substrates (silica) has been utilized to produce hollow nanostructures [36]. Reaction times required in conventional methods are often several days, but the dramatic reduction in the reaction time can be a great benefit of ultrasonic irradiation. A number of different arrangements of equipment have been used for the introduction of the ultrasound irradiation into the chemical reaction systems. The simplest setup for ultrasonic irradiation is the introduction of an ultrasonic horn (often referred to as an ultrasonic probe) directly into an ultrasonic cleaning bath. A typical laboratory-scale sonochemical apparatus consists of a high-intensity ultrasonic titanium horn driven by a piezoelectric transducer, which is directly introduced into a thermostated glass reactor having gas inlets, outlets and reactant inlet with magnetic stirrer and thermo couple [37].The present study describes ultrasonic irradiation process, which promotes the homogeneity of the hydrolysis of ZrO2 and SiO2 precursors, prevents agglomeration during sol–gel reaction to prepare highly efficient sulfated zirconia /silica catalysts i.e. a shell of SZ nanocrystals growing on a core of silica nanoparticles (NSZS) so as to compare with other binary SZ/SiO2 composite catalysts.
2. Materials and Methods
2.1. Reagents and Materials
- Tetraethoxysilane (TEOS 98%), Zirconium n-propoxide [Zr(OC3H7)4] (70 wt.% isopropanol), was procured from Sigma–Aldrich; THF, Conc.H2SO4, Ethanol, Isobutane, and Butene-1were from Merck, India and were used as such.
2.2. Sonochemical Apparatus
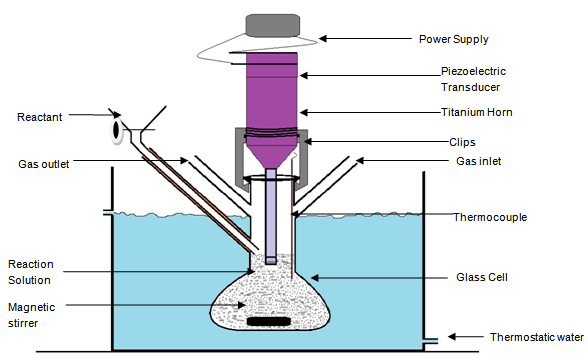
- An ultrasonic irradiation system UP 200S from Hielscher ultrasonic Gmblt (Figure-1) was used to perform the synthesis. The ultrasonic processes operate at 200W and 24 kHz frequency. The amplitude for the reaction was adjustable at 100%. Ultrasonic titanium horn S7 with a diameter of 7 mm and length of 100 mm driven by a piezoelectric transducer was used and is directly introduced into a thermostated glass reactor having gas inlets, outlets, magnetic stirrer and thermocouple with a reactant inlet tube . The titanium horn immersed into the reaction solution, for samples from 20 ml up to 500 ml was used to transmit the ultrasound into the liquid.
2.3. Catalyst Synthesis
- The synthesis was carried out in sonochemical semi- batch glass reactor setup (Figure-1) under ultrasonic irradiation with Ar flow. The preparation of the core-shell NSZS was carried out via a single step synthesis by mixing all the reagents in glass reactor. THF (20.76 g), Double distilled water (6.40 g)and H2SO4(12M, 4.5 g )were mixed in semi batch glass reactor, Tetraethoxysilane (30.16 g)was then added drop wise for over 10 min, under ultrasonic irradiation (200W and 24 kHz )with continuous stirring at 40°C. Ethanol and 10 gm Zirconium n-propoxide (Zr (OC3H7)4 70 wt % in propanol) was then added to adjust the molar ratio of Zr/Si to the desired value and the mixture is stirred for 10 minutes. A slurry was formed which is irradiated at 20°C with a high-intensity 200W and 24 kHz frequency ultrasonic waves by an ultrasonic horn for 10 minutes under argon flow. The amplitude for the reaction was adjustable at 100%.The samples were filtered by vacuum filtration and then dried at room temperature overnight, followed by heating at 100°C for 10 h, calcined at 600℃ for 5h.Extrudates of NSZS were prepared by mixing with alumina binder in a 60:40 ratio, followed by drop wise addition of 3 vol. % glacial acetic acid solution and allowing them to peptize for 2 h. The resultant paste was extruded to 1 mm diameter by extruder, that were dried at room temperature overnight, followed by heating at 100℃ for 12 h. All the extrudates were calcined at 600℃ for 5 h. The calcined extrudates were stored in desiccator for catalytic reactions.
2.4. Catalyst Characterization
2.4.1. X-ray Powder Diffraction (XRD) Studies
- The crystalline phase formed and the crystallinity of sulfated zirconia after calcination at 600℃ was measured by X-ray powder diffractometer (model RigakuDmax-IIIB). The measurements were conducted in a continuous 2θ scan refraction mode using Cu Kα/40 kv/30 mA radiation (λ = 1.54056A°). The samples were scanned in 2θ range of 0–60 degree at a scanning rate of 4.0 deg./min. Crystallite size of tetragonal phase was determined from the characteristic peak (2θ = 30.150 for the (1 1 1) reflection) by using Scherrer formula with a shape factor (K) of 0.9 as below:Crystallite size = K.λ /W. Cos θWhere, W = Wb−Ws; Wb is the broadened profile width of experimental sample and Ws is the standard profile width of reference silica sample.
2.4.2. FT-IR Spectroscopic Studies
- The nature of bonding of sulfate ions with zirconia surface after calcination at 600℃ was studied by FT-IR spectrophotometer (Perkin-Elmer GX). The spectra were recorded in the range 400–4000 cm−1 with a resolution of 4 cm−1 as KBr pellets. The spectra of the samples, diluted with KBr (similarly as in KBr pellets), were recorded at room temperature and after in situ heating at 450℃ at a heating rate of 25℃ min−1. The samples were kept at 450℃ for 30 min, allowing sufficient time for water vapour desorption. Typically 30 scans were co-added at a resolution of 4 cm−1 under dry N2 flow (30 cm3 min−1).
2.4.3. Thermal Analysis
- Thermo gravimetric/differential thermal analysis (TG / DTA) of zirconia sample, before calcination, was carried out by Mettler Toledo (TGA/SDTA 861, Switzerland) using stare software. The samples were heated in a temperature range of 50-1000℃ at the heating rate of 10 °Cmin-1under N2atmosphere.
2.4.4. Surface Area, Pore Volume and Pore Size Distribution
- Specific surface area, pore volume and pore size distribution of sulfated zirconia samples calcined at 600℃ were determined from N2 adsorption–desorption isotherms at 77K by pulse chemisorption (chemisoftTpx V1.02 Micromeritics). Surface area was calculated by using BET equation; pore volume and pore size distribution were calculated by BJH method. The samples were degassed under vacuum at 120℃ for 4 h, prior to adsorption measurement to evacuate the physiosorbed moisture.
2.4.5. Acidity Measurement
- Acidity was characterized by SETARAM C-80 heat flow microcalorimeter which was attached to a volumetric adsorption unit for probe delivery. About 0.1 g of catalyst was outgassed at 723 K under vacuum. The microcalori- metric measurement of ammonia adsorption was carried out at 448 K. Differential heat of ammonia adsorption was determined by introducing small quantities of ammonia on to the out-gassed sample, till the neutralization of all acid sites occurred on the catalyst surface. The heat of adsorption evolved for each dose was calculated from the resulting thermograms and the amount of ammonia adsorbed was calculated from the pressure change.
2.4.6. Scanning Electron Microscopy
- Scanning Electron Microscopy (SEM) was used for high magnification imaging and elemental analysis. A Jeol JSM-6400 scanning electron microscope equipped with an energy dispersive spectrometer (EDS) was used for the analysis. In the pretreatment stage, flat pieces of fresh and aged catalysts were cut, and either potted in epoxy or fastened with a carbon tape in order to obtain side or top views of the catalyst respectively. Catalysts were polished down to 1 µm using diamond paste coating prior to the analysis to avoid the accumulation of charge. The accelerating voltage and current in the measurements were 15 kV and 12 nA respectively, and the resolution of the instrument was 3.5 nm (35 kV).
2.5. Catalyst Activity
- The catalytic performance of the samples was evaluated by using HP Gas chromatograph equipped with thermal conductivity and flame ionization detectors. The catalytic alkylation of isobutane with 1-butene was investigated at 2MPa by using a stainless-steel apparatus equipped with continuous plug flow reactor. A typical reaction was performed with NSZS (1 g), isobutene/1-butene (11:1 mol/mol), and weight hourly space velocity (WHSV) of 4.5 h-1 at 25℃. The catalyst was mixed with the same weight of 50-70 mesh quartz sand and packed into a 3/8' o.d. stainless steel tubular reactor. Quartz sand was packed before and after the catalyst bed. Before the reaction, the reactor system was purged with He and pressurized. Isobutane was flown into the reactor and heating started. The feed was switched from an isobutane to isobutane/butene mixture at least 10 min after a stable reaction temperature was reached. Time-on-stream (TOS) was recorded as zero min at the moment of the feed switch. Online GC analysis with FID detector was used periodically to analyse product composition.
3. Results and Discussion
3.1. Crystalline Phase and Crystallite Size
- X-ray diffraction pattern of NSZS sample after calcination at 600℃ (Fig. 2), exhibits only tetragonal phase with 2θ = 30.15[(111) reflection], 35.15, 50.20 and 59.30. X-ray diffraction data shows that NSZS sample has crystalline size of 8 nm which is lower than non-nano dispersed SZ/SiO2 catalysts reported [38].
3.2. FT-IR Studies
- The FT-IR spectra of sulfated zirconia (NSZS) calcined at 600℃ (Fig.3), gives a broad peak at 3400 cm−1(not shown) and an intense peak at 1632 cm−1which may be attributed to the adsorbed water molecule in the form of stretching and bending mode respectively, associated with zirconia and sulfate group. IR bands of SO42−group in the region of 1200–900 cm−1, with peaks at 1244, 1145, 1078, 1045 and 995 cm−1are characteristic of inorganic chelating bidentate sulfate , assigned to asymmetric and symmetric stretching frequencies of S-O and S-O bonds. The partially ionic nature of the S-O bond is responsible for the Brønsted acid sites in sulfated zirconia samples [39].
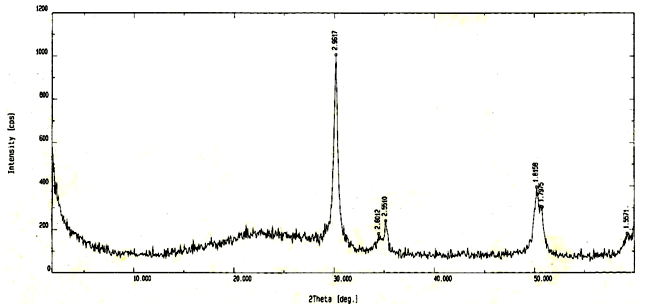 | Figure 2. XRD of sulfated zirconia over silica (NSZS) |
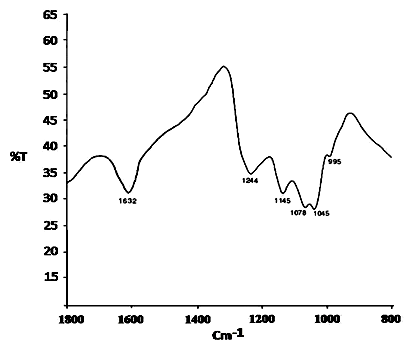 | Figure 3. FT-IR of NSZS catalyst |
3.3. Thermal Analysis
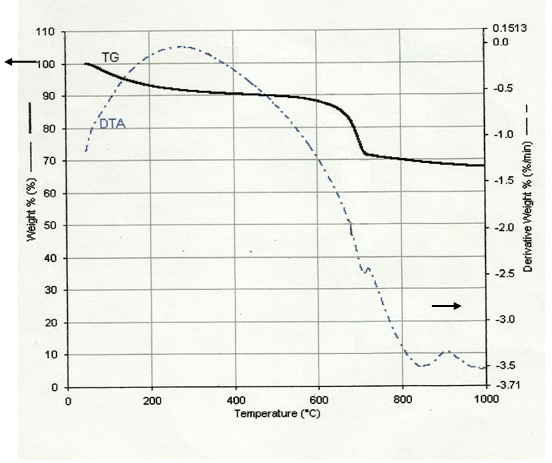
- The TG/DTA curves of the non-calcined NSZS as shown in figure-4 suggests that the optimum temperature for calcination of NSZS sample is 600–650°C where no loss of sulfate species could be observed. The TGA curves show two major mass losses. The first one at about 100°C is accompanied by an endothermic effect. It corresponds to the removal of physiosorbed water and the broadness up to 400°C indicates heterogeneity of strong acid sites. The second weight loss occurs near 550°C and is observed up to 700℃ together with an exothermic peak. The maximum weight loss in the range of 25-30 % is probably due to the decomposition of organic compounds.
3.4. Textural Properties
- The N2 adsorption/desorption isotherms are shown in Figure- 5, classified as type I characteristic of the highly microporous materials with Brunauer-Emmett-Teller (BET) surface area of the calcined sample was 288 m2g-1. The pore size distribution in NSZS is 5 to 27 nm with Single pore volume 0.63 mlg-1, and the average pore diameter is 7.2 nm estimated from their respective adsorption isotherms data. These textural properties of NSZS clearly indicate that ultrasonic method of synthesis is better than conventional method, provide high surface area nano sulfated zirconia core-shell catalyst.
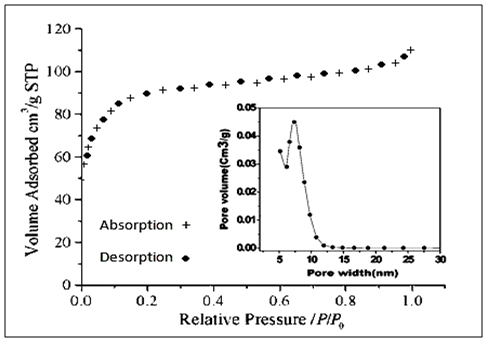 | Figure 5. N2 adsorption isotherm of NSZS catalyst and pore size distribution (inset) |
3.5. Scanning Electron Microscopy
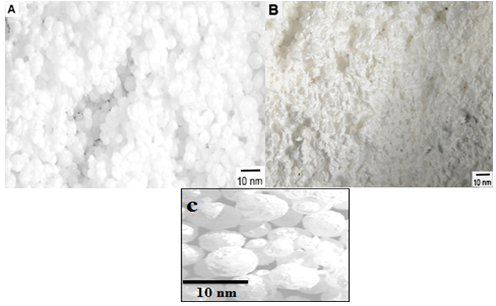 | Figure 6. SEM of NSZS catalyst Ultrasonic irradiation applies for (A) 20 min.,(B) 40 min. and (C) high resolution |
- The surface morphology of the zirconia samples prepared by ultrasonic irradiation method was found to be different from other conventional methods (Figure-6). The NSZS catalyst prepared by ultrasonic irradiation was observed to have spherical-shaped particles. Ultrasonic irradiation applied for 20 minutes gave well shaped particles (figure-6 A) while in case of radiation exposed for 40 minutes, particles are not well shaped (figure-6 B), and are assumed to be high energy cavitation for long time which promotes the collapse of particles.
3.6. Catalytic Activity
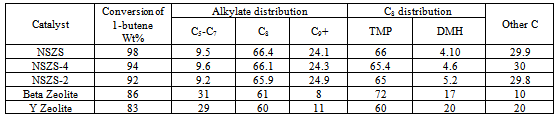
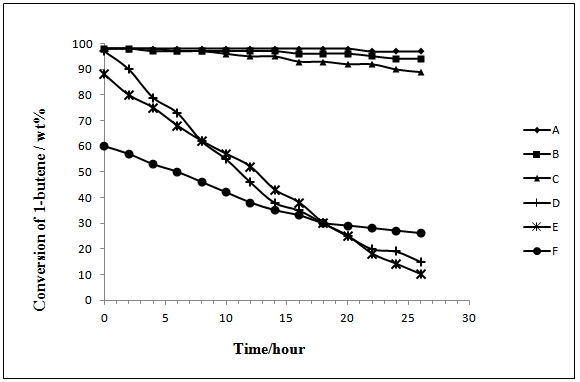
- The 1-butene conversion and product distribution obtained at 25℃ after 1 hour of alkylation reaction of isobutane on NSZS,Y zeolite and Beta Zeolite catalysts are summarized in Table 1. The conversion with NSZS catalyst 98% is higher than Beta Zeolite 86% and Y Zeolite 83%. Catalyst regenerated four times (NSZS-4) and two times (NSZS-2) with calcination and sulfonation shows 94% and 92% conversion respectively which is higher than fresh Beta Zeolite and Y Zeolite catalyst. The selectivity of trimethyl- pentanes (TMPs) is 66%, 72% and 60% over NSZS, Beta Zeolite and Y Zeolite respectively, demonstrates that the selectivity of NSZS is higher than both these catalysts. NSZS exhibited a higher catalytic activity in many other reactions over a longer operation time than other catalysts.The alkylation reaction of isobutane with 1-butene showed that NSZS (Zr/Si=40:60 mol/mol) retained an activity of 96% after catalytic reaction for 26 h. Even after being regenerated four times, it kept its activity at over 94%. In a sharp contrast, the Beta Zeolite and Y Zeolite showed very high activities only within the first 2 h of the reaction, and then lost their activity quickly.The high activity shown by the NSZS catalyst with its unique core–shell structure and high dispersion of SZ nanocrystals on the surface of silica matrix could be attributed to the sonochemical method of synthesis. This allows for the easy diffusion of the reactants and oxygen that leads to the lowest carbon deposition and coke formation.
|
4. Conclusions
- The core-shell Sulfated zirconia over silica (NSZS) catalyst prepared by ultrasonic irradiation i.eSonochemicalsynthesis, which promotes the homogeneity of the hydrolysis of ZrO2 and SiO2 precursors, prevents agglomeration during sol–gel reaction and keeps its stability during the catalytic reaction over a long period of time, even after regeneration by calcination and sulfation many times. Structural and textural properties of NSZS showed improvement in terms of sulfur loading, small crystalline size, surface area, pore volume, efficiency, regeneratability, catalytic activity and stability over a long period of reaction time and as such would have a great potential for practical applications in solid catalytic reactions. The ultrasonic irradiation technique can be readily applied to the synthesis of other silica-supported metals or metal oxides, such as TiO2/SiO2, CuO/SiO2, Pt/SiO2, Au/SiO2, and so forth, for various industrial catalytic reactions. Thus the ultrasonic irradiation process used to prepare highly efficient sulfated zirconia /silica catalysts with a shell of SZ nanocrystals growing on a core of silica nanoparticles, maybe considered a better technique in comparison to commonly used simple calcination, one step and two step techniques.
ACKNOWLEDGEMENTS
- This work is supported by IFTM University Research fund. Authors thank A.K. Singh Department of Applied Chemistry Division, S.D. Sharma IIC, IIT Roorkee and N.K. Saini, WIHG, Dehradun for help and encouragement.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML