-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2013; 3(1): 14-22
doi:10.5923/j.chemistry.20130301.04
Design, Synthesis and Characterization of Novel 6,7-dimethoxy-N2-(substituted benzyl)-N2-propylquinazoline-2,4-diamine Derivatives as Anxiolytic and Antidepressant Agents
Maneesh Kumar Srivastav1, Md. Shamshuddin2, S. M. Shantakumar1
1Department of Pharmaceutical Chemistry, V. L. College of Pharmacy, Raichur, 584103, Karnataka, India
2Department of Pharmacology, V. L. College of Pharmacy, Raichur, 584103, Karnataka, India
Correspondence to: S. M. Shantakumar, Department of Pharmaceutical Chemistry, V. L. College of Pharmacy, Raichur, 584103, Karnataka, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
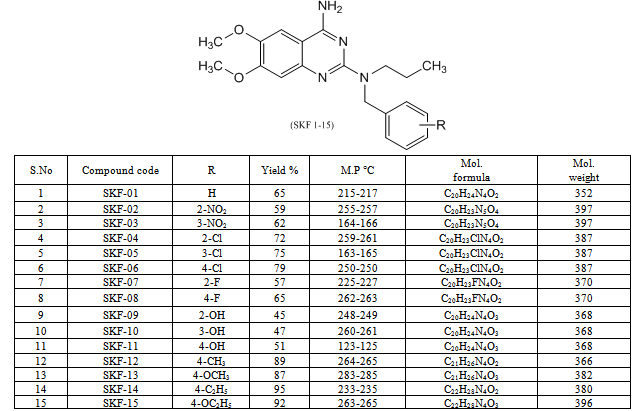
In order to produce new lead for anxiolytic and antidepressant drug, a new series of quinazoline analogues was designed to mimic ATC-0175 structural features and fitted with functional groups believed to enhance anxiolytic and antidepressant activity. The synthesized compounds were evaluated for anxiolytic and antidepressant activity by elevated plus-maze and tail-suspension method. The targeted compounds (SKF 1-15) were synthesized from 2-chloro-6,7-dimethoxy quinazolin-4-amine and N-(substituted benzyl) propan-1-amines in isoamyl alcohol medium under reflux for 24 h. All the targeted compounds are new chemical entities and obtained in satisfactory yields (Table 1). The structures of synthesized compounds are in good agreement with their corresponding spectral data.
Keywords: Quinazoline, Anxiolytic Activity, Antidepressant Activity, MAO-A, MAO-B Inhibition
Cite this paper: Maneesh Kumar Srivastav, Md. Shamshuddin, S. M. Shantakumar, Design, Synthesis and Characterization of Novel 6,7-dimethoxy-N2-(substituted benzyl)-N2-propylquinazoline-2,4-diamine Derivatives as Anxiolytic and Antidepressant Agents, American Journal of Chemistry, Vol. 3 No. 1, 2013, pp. 14-22. doi: 10.5923/j.chemistry.20130301.04.
Article Outline
1. Introduction
- Serotonin, released from nerve terminals in virtually all regions of the brain, plays a direct role in normal functions of the central nervous system and in mental disease such as anxiety, depression, psychosis, obsessive compulsive disorders, aggression, and eating disorders. The role of serotonin receptor in the brain is being actively studied with respect to normal and abnormal functions of the brain. Fourteen different serotonin receptors are known. Serotonin receptor dysfunction is implicated in many neuropsychiatric disorders[1-9]. Serotonins are metabolized by an enzyme called monoamine oxidase (MAO). The inhibitors of monoamine oxidase have shown therapeutic value in a variety of neurodegenerative diseases[10]. The discovery in the 1950s of the antidepressant properties of MAO inhibitors (MAOIs) was the major finding that led to the monoamine theory of depression. Earlier MAO inhibitors introduced into clinical practice for the treatment of depression were abandoned due to adverse side-effects, such as hepatotoxicity and orthostatic hypotension. This handicap was thought to be related to nonselective and irreversible monoamine oxidase inhibition. However, more recently a better knowledge of an enzyme, in particular to the identification of two isomeric forms (MAO-A and MAO-B) was reported. These two forms of MAO are characterized by their different sensitivities to inhibitors and their different specificities to substrates[11]. MAO-A preferably metabolizes serotonin, adrenalin, and noradrenalin[12], whereas β-phenyl ethylamine and benzylamine are predominantly metabolized by MAO-B[13] Tyramine, dopamine and some other important amines are common substrate for both isoenzymes[14].MAO-A inhibitors have been used mostly in the treatment of mental disorder, in particular depression and anxiety [15-17] while MAO-B inhibitors could be used in the treatment of Parkinson’s disease and perhaps, Alzheimer’s disease[18,19]. Despite the considerable progress in understanding the interaction of two enzyme forms with their preferred substrate and inhibitors, few general rule are yet available for the rational design of potent and selective inhibitors of MAO-A and MAO-B. Various efforts have been oriented towards the discovery of reversible and selective inhibitor of MAO-A/MAO-B leading to a new generation of compounds. Moreover, a number of quinazolinones and their analogues have shown clinical importance through exhibition of monoamine oxidase inhibitor activity. It was observed that the 2nd and 3rd position of quinazolines have been target for the substitution of deferent heterocyclic moieties[20-24]. The quinazoline molecule as a promising anxiolytic and antidepressant activity have yet to be launched except some drug candidate like ATC-0175[25], none of the other derivatives of quinazoline had come to the development phase.
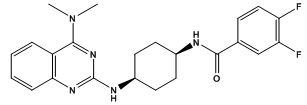 | Figure 1. An example of anxiolytic and antidepressant drug candidate (ATC-0175) |
2. Experimental
- The reagents and starting material 2-chloro-6, 7-dimethoxyquinazolin-4-amine was purchased from Sigma-Aldrich Company and used without further purification. Melting points of the synthesized compounds were determined by using an open capillary tube method and are uncorrected. IR spectra (Vmax in cm-1) were recorded on a Shimadzu FT-IR 8300 Spectrophotometer using KBr pellets technique. 1HNMR Spectra were recorded using Bruker avance II-400 spectrophotometer using DMSO.d6 as the solvent and TMS as the internal reference (chemical shifts in ppm). The purity of products was checked by TLC using silica gel G60 (Merck, Germany) plates in n-hexane-ethyl acetate (7:3) solvent system for all the new synthesized compounds and spots were visualized under UV light.
2.1. Synthesis of N-(substitutedbenzyl) propan-1amine (F 1-15)
- The appropriate substituted / unsubstituted benzaldehyde (3.33 mmol) was dissolved in anhydrous methanol (20 mL) and was cooled to oC under stirring. To this cold mixture n-propylamine (0.39 mL, 6.66 mmol) was added drop wise and stirring was continued for 30 min at oC, then for 3 h at ambient temperature. The reaction mixture was again cooled to oC and added NaBH4 (0.19 g, 5.0 mmol) in three portions at 10 min intervals. Then it was stirred at room temperature for another 1 h and the solvent was evaporated to 1/3 of its original volume under vacuum. Cold water (5 mL) and NaHCO3 (0.28 g, 3.33 mmol) were added to the above concentrated solution, stirred for 10 min and extracted with dichloromethane (3x20 mL). Combined organic phase was dried over Na2SO4 and evaporated the solvent and dried under vacuum to get the compounds (F 1-15).
2.2. General Procedure for the Synthesis of 6,7-dimethoxy-N2-(substitutedbenzyl)-N2-propylquinazoline-2,4-diamines (SKF 1-15)
- A mixture of 2-chloro-6,7-dimethoxyquinazolin-4-amine (1.0 g, 4.17 mmol) and appropriate N-(substituted benzyl) propan-1-amine (F1-15) (6.26 mmol) was refluxed in isoamylalcohol (50 mL) for 24 h (all the reactions were monitored by TLC using n-hexane: ethyl acetate (7:3) as eluents). After completion of reaction, excess solvent was removed in vacuum and resulting residue was washed with acetone to afford colorless white crystals which were recrystallized from aqueous ethanol. 1). Compound SKF-01:IR (KBr, cm-1): 3385 (N-H stretching), 2923 (Ar-CH stretching), 1478 (C=N). 1H NMR (DMSO.d6) δ: 0.6-0.8 (t, 3H of CH3), 1.5-1.7 (m, 2H of CH2), 2.5-2.7 (t, 2H of CH2), 3.3-3.9 (m, 6H of 2-OCH3), 4.7 (s, 2H of N-CH2), 7.0-7.7 (m, 7H of Ar-H), 8.1 (s, 1H of NH), 9.3 (s, 1H of NH). MS (m/z): M+1= 354.2). Compound SKF-02:IR (KBr, cm-1): 3365 (N-H stretching), 2920 (Ar-CH stretching), 1485 (C=N). 1H NMR (DMSO.d6) δ: 0.7-0.9 (t, 3H of CH3), 1.7-1.9 (m, 2H of CH2), 2.8-3.1 (t, 2H of CH2), 3.5-4.1 (m, 6H of 2-OCH3), 4.9 (s, 2H of N-CH2), 7.3-8.1 (m, 6H of Ar-H), 8.5 (s, 1H of NH), 9.6 (s, 1H of NH). MS (m/z): M+1= 399.3). Compound SKF-03:IR (KBr, cm-1): 3378 (N-H stretching), 2933 (Ar-CH stretching), 1475 (C=N). 1H NMR (DMSO.d6) δ: 0.6-0.8 (t, 3H of CH3), 1.6-1.9 (m, 2H of CH2), 2.6-3.0 (t, 2H of CH2), 3.3-3.9 (m, 6H of 2-OCH3), 4.7 (s, 2H of N-CH2), 7.2-8.0 (m, 6H of Ar-H), 8.3 (s, 1H of NH), 9.2 (s, 1H of NH). MS (m/z): M+1= 399.4). Compound SKF-04:IR (KBr, cm-1): 3385 (N-H stretching), 2918 (Ar-CH stretching), 1498 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.6-1.7 (m, 2H of CH2), 3.7-3.9 (t, 2H of CH2), 3.8-4.1 (m, 6H of 2-OCH3), 4.8 (s, 2H of N-CH2), 7.4-7.9 (m, 6H of Ar-H), 8.4 (s, 1H of NH), 9.6 (s, 1H of NH). MS (m/z): M+2= 389.5). Compound SKF-05:IR (KBr, cm-1): 3380 (N-H stretching), 2920 (Ar-CH stretching), 1477 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.4-1.6 (m, 2H of CH2), 3.4-3.6 (t, 2H of CH2), 3.8-4.1 (m, 6H of 2-OCH3), 4.7 (s, 2H of N-CH2), 7.3-7.9 (m, 6H of Ar-H), 8.2 (s, 1H of NH), 9.3 (s, 1H of NH).MS (m/z): M+2= 389.6). Compound SKF-06:IR (KBr, cm-1): 3395 (N-H stretching), 2926 (Ar-CH stretching), 1465 (C=N). 1H NMR (DMSO.d6) δ: 0.9-1.0 (t, 3H of CH3), 1.5-1.7 (m, 2H of CH2), 3.6-3.7 (t, 2H of CH2), 3.9-4.1 (m, 6H of 2-OCH3), 4.9 (s, 2H of N-CH2), 7.2-7.7 (m, 6H of Ar-H), 8.3 (s, 1H of NH), 9.3 (s, 1H of NH). MS (m/z): M+2= 389.7). Compound SKF-07:IR (KBr, cm-1): 3363 (N-H stretching), 2926 (Ar-CH stretching), 1465 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.6-1.7 (m, 2H of CH2), 3.5-3.7 (t, 2H of CH2), 3.8-4.1 (m, 6H of 2-OCH3), 4.8 (s, 2H of N-CH2), 7.3-7.9 (m, 6H of Ar-H), 8.4 (s, 1H of NH), 9.3 (s, 1H of NH). MS (m/z): M+1= 372.8). Compound SKF-08:IR (KBr, cm-1): 3380 (N-H stretching), 2921 (Ar-CH stretching), 1475 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.7-1.8 (m, 2H of CH2), 3.6-3.9 (t, 2H of CH2), 3.9-4.2 (m, 6H of 2-OCH3), 4.9 (s, 2H of N-CH2), 7.4-8.1 (m, 6H of Ar-H), 8.3 (s, 1H of NH), 9.5 (s, 1H of NH). MS (m/z): M+1= 372.9). Compound SKF-09:IR (KBr, cm-1): 3435 (OH stretching), 3385 (N-H stretching), 2923 (Ar-CH stretching), 1467 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.6-1.7 (m, 2H of CH2), 3.5-3.8 (t, 2H of CH2), 3.8-4.2 (m, 6H of 2-OCH3), 4.8 (s, 2H of N-CH2), 7.3-8.1 (m, 6H of Ar-H), 8.4 (s, 1H of NH), 9.5 (s, 1H of NH), 10.1 (s, 1H of OH). MS (m/z): M+1= 369.10). Compound SKF-10:IR (KBr, cm-1): 3445 (OH stretching), 3365 (N-H stretching), 2926 (Ar-CH stretching), 1460 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.5-1.6 (m, 2H of CH2), 3.4-3.6 (t, 2H of CH2), 3.6-3.9 (m, 6H of 2-OCH3), 4.6 (s, 2H of N-CH2), 6.9-7.7 (m, 6H of Ar-H), 8.1 (s, 1H of NH), 8.9 (s, 1H of NH), 10.2 (s, 1H of OH). MS (m/z): M+1= 369.11). Compound SKF-11:IR (KBr, cm-1): 3425 (OH stretching), 3375 (N-H stretching), 2925 (Ar-CH stretching), 1473 (C=N). 1H NMR (DMSO.d6) δ: 0.8-0.9 (t, 3H of CH3), 1.6-1.7 (m, 2H of CH2), 3.7-3.9 (t, 2H of CH2), 3.9-4.1 (m, 6H of 2-OCH3), 4.8 (s, 2H of N-CH2), 7.3-8.1 (m, 6H of Ar-H), 8.3 (s, 1H of NH), 9.5 (s, 1H of NH), 10.5 (s, 1H of OH). MS (m/z): M+1= 369.
|
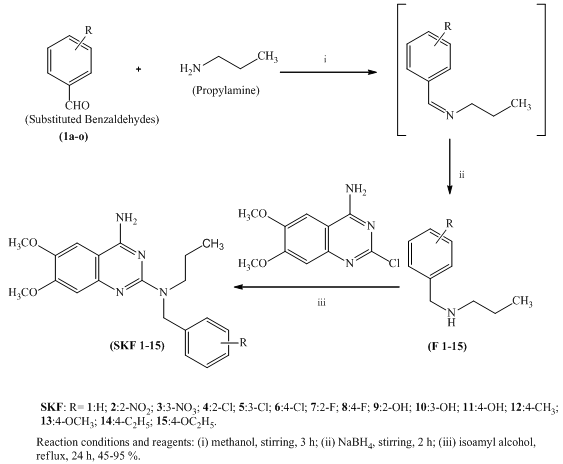 | Scheme 1. The synthesis of 6,7-dimethoxy-N2-(substituted benzyl)-N2-propylquinazoline-2,4-diamine derivatives (SKF 1-15) |
3. Pharmacological Activity
3.1. Anxiolytic and Antidepressant Activity
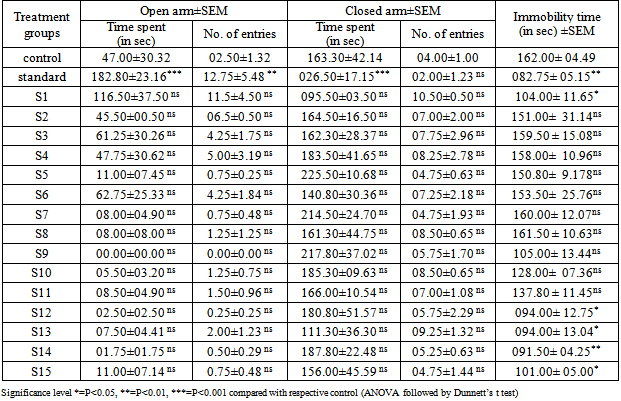
- The anxiolytic and antidepressant activity of synthesized compounds was carried on albino mice of either sex weighing 20-25 g. A plus-maze test was applied for anxiolytic activity[26] and antidepressant activity was tested by tail-suspension method[27]. Each animal was used for the plus-maze at first and then for the tail-suspension test. All experiments for animal testing were carried in accordance with the guidelines of Institutional Animal Ethics Committee. The animals were housed six per cage and kept in room with controlled temperature (20 + 2°C) and 12 h light and dark cycle. All animals were allowed libitum access to food and water; all compounds were dissolved in DMSO/water (1:4) and injected to animal intraperitonealy at 200 mg/kg doses in approximately 0.1 mL volume 1 h before the test. Diazepam (10 mg/kg) and Imipramine (15 mg/kg) were used as standard reference for anxiolytic and antidepressant activity respectively. 0.1 mL DMSO/water (1:4) was given to the control animals.
3.2. Elevated Plus-maze Test
- The elevated plus-maze is used to determine the mouse’s unconditioned response to a potentially dangerous environment and anxiety related behaviour is measured by the degree to which the mouse avoids the unenclosed arms of the maze. The test is useful in testing the effect of anxiolytic drugs.It is a standard test of fear and anxiety in which the animal is placed in the canter of an elevated four arm maze in which two arm are open (50×10 cm) and two are enclosed (50×10×40 cm) with an open roof. The two open arms are opposite to each other. The maze was elevated to a height of 50 cm. the measures indicated by in the procedure section were taken by two observers sitting in the same room with the maze.Mice were placed in the centre of the maze and the following measures scored by two observers for 5 minutes. The number of times the animal enters each of the arms and the time spent in each arms is noted[26]. Two indices of anxiety are obtained, the amount of time spent in closed arm, open arm and the number of entries into the arms. The test is rapid and to be sensitive to the effect of anxiolytic and anxiogenic agents. Anxiolytic agents decreasing and anxiogenic agents increasing the amount of time spent in closed arms, whereas anxiolytic agents are increasing and anxiogenic agents are decreasing the number of entries[28, 29].
3.3. Tail-suspension Method
- The tail-suspension method is used to evaluate antidepressant activity of drugs. In this test immobility displayed by mice, when subjected to an unavoidable and inescapable stress has been hypothesized to reflect behavioural despair which in turn may reflect depressive disorder in humans. Clinically antidepressants reduce the immobility that mice display after active and unsuccessful attempts to escape when suspended by tail.Individual mice are suspended on the edge of a shelf 58 cm above a table top by adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility is recorded for a period of 5 minutes. Mice are considered immobile when they hang passively and completely motionless for at least 1 minute.Statistical analysis of results were expressed as mean ± SEM and evaluated by one way ANOVA followed by Dunnett’s t test.
|
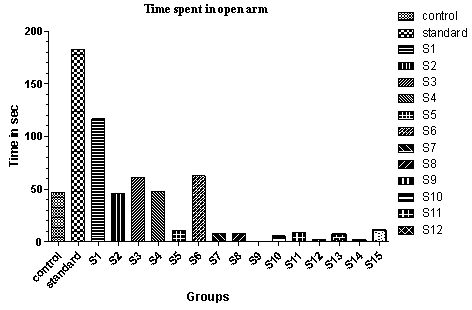 | Figure 2. Effect of various drugs treatment on antianxiety activity by elevated plus-maze method |
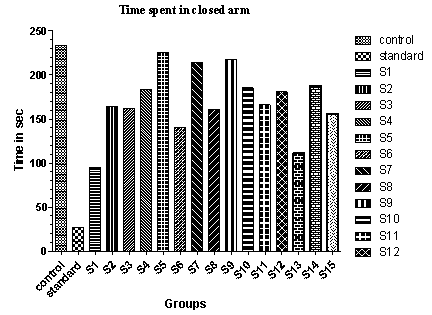 | Figure 3. Effect of various drugs treatment on antianxiety activity by elevated plus-maze method |
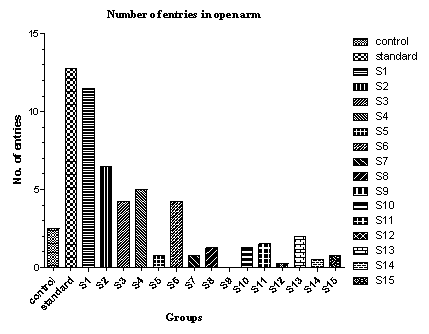 | Figure 4. Effect of various drugs treatment on antianxiety activity by elevated plus-maze method |
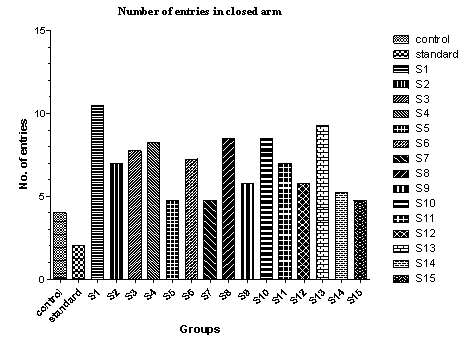 | Figure 5. Effect of various drugs treatment on antianxiety activity by elevated plus-maze method |
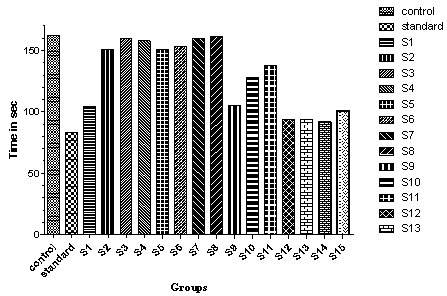 | Figure 6. Effect of drugs treatment on immobility time in tail-suspension method in mice |
4. Result and Discussion
- The synthetic approach for the title compounds were depicted in scheme-1, the yields were in between 45-95 %. The spectral data of synthesized compounds was in agreement with the assigned structure. The title compounds were evaluated for their anxiolytic and antidepressants activities and results are tabulated in table 2. A substantial number of compounds have been identified exhibiting moderate to excellent antidepressant activity in comparison to Imipramine (standard drug used for the study). Our findings suggest that selectivity for this activity is primarily dependent on the presence of substituent on the phenyl ring which is link by a tertiary amine group attached to the 2nd position of quinazoline ring. These results suggest that the presence of electron withdrawing groups like –Cl, F and NO2 on phenyl ring at ortho, meta and para position i. e. compound SKF-02, SKF-03, SKF-04, SKF-05, SKF-06, SKF-07, SKF-08, are decreasing the activity in comparison to compounds without any substitution (SKF 01). However, it is noteworthy that the presence of OH group (SKF-09) at ortho position producing a marked antidepressant activity while change in the position of –OH group from the ortho position to meta or para position (SKF-10, SKF-11) of the phenyl ring decreases the potency of the compounds towards the antidepressant activity. While electron releasing groups like - CH3, -OCH3 -C2H5 and -OC2H5 at para position (SKF-12, SKF-13, SKF-14 and SKF-15) are more desirable and producing excellent results. It did not appear surprising to us that these antidepressants molecule do not display anxiolytic properties, in comparison with Diazepam (standard drug used for the study) as anxiolytic. Conversely, it was more curious to note that the same molecules were inversely producing anxiogenic activity. We have here by generated a novel molecular framework, 6,7-dimethoxy- N2-(substituted benzyl)-N2-propylquinazoline-2,4-diamines by utilizing the chemical lead based approach and concept of bio-isosterism. Compounds based on this framework have found to be as antidepressant agents. These results may open up new avenues in designing candidate acting on more than one rate limiting step. Of the compounds tested, Compound SKF-12, SKF-13, SKF-14, and SKF-15 clearly proved the most promising in term of antidepressant activity (potency). This lead molecule SKF-12, SKF-13, SKF-14, and SKF-15 could be further utilized for designing newer antidepressant agents.
ACKNOWLEDGEMENTS
- The authors are thankful to the management of V. L. College of Pharmacy, Raichur for providing the necessary facilities to carry out the research work. The authors are highly indebted to the SAIF Punjab University, Chandigarh and Oxygen Health Care Research Pvt. Ltd. Ahmadabad for providing NMR, LCMS and IR Spectra’s.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML