-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(6): 347-354
doi:10.5923/j.chemistry.20120206.09

Synthesis, Structure Elucidation and Application of Some New Azo Disperse Dyes Derived from 4-Hydroxycoumarin for Dyeing Polyester Fabrics
Moaz M. Abdou 1, Samir Bondock 2, El-Sayed I. El-Desouky 2, M. A. Metwally 2
1Egyptian Petroleum Research Institute, Nasr city, P.O. 11727, Cairo, Egypt
2Department of Chemistry, Faculty of Science, Mansoura University, ET-35516, Egypt
Correspondence to: M. A. Metwally , Department of Chemistry, Faculty of Science, Mansoura University, ET-35516, Egypt.
| Email: |  |
Copyright © 2012 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

As a part of our ongoing interest in disperse dyes and task specific azo dyes with better dyeing properties, we synthesized a series of 3-aryl(hetaryl)hydrazono-2,4-chromandiones 4a-k via Coupling of 4-hydroxycoumarin with diazotized aniline derivatives. Structures of the synthesized compounds have been investigated by means of UV, IR, 1H-NMR and mass spectroscopy in order to elucidate their tautomeric and isomeric structures. The results of such spectral data indicated that compounds 4a-k exist predominantly in the hydrazo structure (D) as a mixture of Z and E-isomer with ratio ~ 84.5:15.5. Finally, the prepared dyestuffs 4a-k were applied as disperse dyes for dyeing polyester fabrics and their fastness properties were evaluated. Also, the position of color in CIELAB coordinates (L*, a*, b*, H*, C* and K/S) estimated and discussed.
Keywords: Azo-Hydrazone Tautomerism, Disperse Dyes, Polyester Fabrics, Fastness Properties, CIELAB Coordinates
Cite this paper: Moaz M. Abdou , Samir Bondock , El-Sayed I. El-Desouky , M. A. Metwally , Synthesis, Structure Elucidation and Application of Some New Azo Disperse Dyes Derived from 4-Hydroxycoumarin for Dyeing Polyester Fabrics, American Journal of Chemistry, Vol. 2 No. 6, 2012, pp. 347-354. doi: 10.5923/j.chemistry.20120206.09.
Article Outline
1. Introduction
- Disperse dyes are very popular and important class of dyes for dyeing polyester fabrics owing to their brilliancy, wide range of hue and excellent fastness properties[1]. The majority of disperse dyes are azodyes due to the ease with which an extra ordinary number of molecular combinations can be generated by varying the diazo and coupling components and they provide a very wide color gamut of high color strength[2-4]. Especially useful in this respect are azodyes derived from coupling of diazonium salts with 4-hydroxycoumarin as coupling component. These azodyes are very useful as precursors for the synthesis of heterocyclic ring systems, which play an important role in pharmaceutical chemistry and dyestuff industry[5,6]. Another interesting feature of this class lies on its tautomeric behavior whichhas been studied by several investigators for many years. They were originally regarded as azo-structure (A)[7,8], but others workers[9-11] tended to the view that they were keto-hydrazone structure (D). Recently, two groups of workers[12,13] proposed that compounds exist as a mixture of tautomeric forms(A) and (D) (Fig. 1). Based on these results, the approach reported here is an extension and a continuation of our interest in the synthesis and elucidation of the tautomeric structures of mono and bisazo dyes which were able to dye polyester fabrics [5,6,14-16]. Thus, this work is aiming to study the synthesis of a series of azo disperse dyes having different substituted groups 4a-k in a trial to provide a better understanding of the prevalent tautomeric structure of the compounds in question. Moreover, the prepared dyestuffs will be dyed on polyester fabrics and their dyeing properties, light fastnesses, wash fastnesses, sublimation fastnesses and the position of color in CIELAB coordinates (L*, a*, b*, H*, C*) were evaluated.
2. Experimental Section
2.1. Materials and Instrumentation
- General: Elemental analyses were performed at the Microanalytical Center, Cairo University, using CHNS-932 (LECO) Vario Elemental Analyzers and the results were within the accepted range (±0.40) of the calculated values. IR spectra were obtained using a Mattson 5000 FTIR spectrometer (ν, cm-1), using samples in KBr disks and only partial spectral data are listed. The Ultraviolet absorption spectra were recorded in the range 200-800 nm on Shimadzu 700 spectra photometer in dioxane using a concentration 0f 10-4 mol dm-3. 1H NMR spectra were measured on a Bruker WP300 (300 MHz) spectrometer (δ, ppm), using DMSO-d6 as a solvent and TMS as an internal standard. Mass spectra were recorded on a Finnigan MAT 212 instrument. Scoured and bleached polyester 100% (150:130 g/m2, 70/2 denier) was obtained from El-Shourbagy, Egypt. The fabric was further treated before dyeing with a solution containing 5 g/L nonionic detergent (Hostapal CV, Clariant-Egypt) and 2 g/L sodium carbonate at a liquor ratio 20:1 at 60°C for 30 min, thoroughly washed in water and air dried at room temperature. The dispersing agent Setamol WS was supplied by BASF (Germany). The dyeing assessment, fastness tests, and color measurements were carried out in Laboratories and Research sector in El-Nasr Company for Spinning and Weaving, El-Mahalla El-Kubra, Egypt. The colorimetric measurements (L*, a*, b*, C*, H* and K/S) were carried out using a Gretag Macbeth CE 7000A spectrophotometer (D65 illumination, 10° observer). Fastness to washing was carried out using the automatic launder Rotadyer (sponsored by the British Standard Institute-Society of Dyers and Colorists), fastness to perspiration was assessed according to the test sponsored by the (BSS), fastness to rubbing was carried out according to the standard method of testing (BSS) using Crockmeter of Electric Hungarian FD-17 type, fastness to sublimation was carried out using the Electric Japanese Thermotester T-10 type and fastness to light was carried out using the "Weather-o-meter" (Atlas Electric Devices Co. USA), AATCC standard test method.
2.2. Synthesis and Spectroscopic Characterization
2.2.1. General procedure for the synthesis of 3-aryl (hetaryl) hydrazono-2,4-chromandione derivatives 4a-i
- To a stirred suspension of aromatic amine (0.87 mmol) in water (3 ml), concentrated HCl (2 ml 5 M) was added with heating until complete dissolution of the amine hydrochloride. The resulted solution was cooled at 0°C on ice bath, while some solid started to precipitate. To this mixture, NaNO2 (0.9 mmol, 60 mg) dissolved in a minimum amount of water was added slowly with vigorous stirring. The stirring was continued at low temperature for 15 min. Then, it has been added to an ice-cooled solution of 4-hydroxycoumarin (0.87 mmol) and sodium hydroxide (0.87 mmol) in ethanol (20 ml). The reaction mixture was allowed to stir at (0–5°C) for 2 hrs, and then the solid was collected by filtration. The crude products thus obtained, were dried and recrystallized from acetic acid to give the corresponding compounds 4a-i.
2.2.1.1. 3-(2-Phenylhydrazono)-2,4-chromandione (4a)± (Z(Z)-3-)
- Yellow solid, Yield: 79% UV-Vis (λmax in dioxane): 258, 416 nm. IR (KBr, ímax/cm–1): 3177 (NH), 3019 (CH, Ar.), 1732 (C=O, lactone), 1636 (C=O, C-4), 1611(
2.2.1.2. 3-(2-(4-Methylphenyl)hydrazono)-2, 4-chromandione (4b)
- Yellow solid, Yield: 75% UV-Vis (λmax in dioxane): 252, 422 nm. IR (KBr, ímax/cm–1): 3190 (NH), 3033 (CH, Ar.), 2915 (CH, Aliph.), 1736 (C=O, lactone), 1628 (C=O, C-4), 1604 (
2.2.1.3. 3-(2-(4-Methoxyphenyl)hydrazono)-2, 4-chromandione (4c)
- Yellow solid, Yield: 83% UV-Vis (λmax in dioxane): 256, 436 nm. IR (KBr, ímax/cm–1): 3228 (NH), 3049 (CH, Ar.), 2975 (CH, Aliph.), 1737 (C=O, lactone), 1634 (C=O, C-4), 1609 (
2.2.1.4. 3-(2-(4-Chlorophenyl)hydrazono)-2, 4-chromandione (4d)
- Yellow solid, Yield: 73% UV-Vis (λmax in dioxane): 257, 432 nm. IR (KBr, ímax/cm–1): 3200 (NH), 3038 (CH, Ar.), 1730 (C=O, lactone), 1640 (C=O, C-4), 1614 (
2.2.1.5. 3-(2-(2,4-Dichlorophenyl)hydrazono)-2, 4-chromandione (4e)
- Yellow solid, Yield: 79% UV-Vis (λmax in dioxane): 258, 426 nm. IR (KBr, ímax/cm–1): 3240 (NH), 3059(CH, Ar.), 1730 (C=O, lactone), 1636 (C=O, C-4), 1611 (
2.2.1.6. 3-(2-(4-Bromophenyl)hydrazono)-2, 4-chromandione (4f)
- Yellow solid, Yield: 72% UV-Vis (λmax in dioxane): 258, 431 nm. IR (KBr, ímax/cm–1): 3170 (NH), 3035 (CH, Ar.), 1734 (C=O, lactone), 1638 (C=O, C-4), 1619 (
2.2.1.7. 3-(2-(4-Nitrophenyl)hydrazono)-2,4-chromandione (4g)
- Yellow solid, Yield: 87% UV-Vis (λmax in dioxane): 260, 438 nm. IR (KBr, ímax/cm–1): 3210 (NH), 3046 (CH, Ar.), 1732 (C=O, lactone), 1637 (C=O, C-4), 1615 (
2.2.1.8. 3-(2-(4-diphenyldiazene)hydrazono)-2, 4-chromandione (4h)
- Orange solid, Yield: 79% UV-Vis (λmax in dioxane): 258, 426 nm. IR (KBr, ímax/cm–1): 3199 (NH), 3019 (CH, Ar.), 1731 (C=O, lactone), 1636 (C=O, C-4), 1611(
2.2.1.9. 3-(2-Antipyrinylhydrazono)-2,4-chromandione (4i)±
- Red solid, Yield: 75% UV-Vis (λmax in dioxane): 252, 439 nm. IR (KBr, ímax/cm–1): 3220 (NH), 3080 (CH, Ar.), 2956 (CH, Aliph.), 1740 (C=O, lactone), 1660, 1635 (C=O, C-4/pyrazolone), 1613 (
2.2.2. General Procedure for the Synthesis of Bis Hydrazono Derivatives 4j and 4k
- The corresponding aryl diazonium chloride was prepared by adding cold sodium nitrite solution (1.2 gm in 12 ml H2O) to a cold suspension of p-phenylenediamine and/or benzidine (1.74 mmol) in concentrated HCl (6 ml) with stirring at low temperature for 15 min. Then, it has been added to an ice-cooled solution of 4-hydroxycoumarin (0.87 mmol) and sodium hydroxide (0.87 mmol) in ethanol (20 ml). The reaction mixture was allowed to stir at (0–5 ◦C) for 2 hrs, and then the solid was collected by filtration. The crude products thus obtained, were dried and recrystallized from from ethanol-DMF mixture (1:2) to give the corresponding compounds 4j and 4k.
2.2.2.1. 3,3`-(1,4-dihydrazonobenzene)bis(2, 4-chromandione) (4j)
- Brown solid, Yield: 81% IR (KBr, ímax/cm–1): 3227 (NH), 3019 (CH, Ar.), 1738 (C=O, lactone), 1635 (C=O, C-4), 1610 (
2.2.2.2. 3,3`-(4,4'-dihydrazono biphenyl)bis (2,4-chromandione) (4k)
- Black solid, Yield: 68% IR (KBr, ímax/cm–1): 3240 (NH), 3019 (CH, Ar.), 1725 (C=O, lactone), 1629 (C=O, C-4), 1608 (
2.3. Dyeing and Fastness Determinations
2.3.1. Preparation of Dye Dispersion
- The required amount of the dye (2% shade) was dissolved in 1cm3 acetone and then added dropwise with stirring to a solution of Setamol WS (sodium salt of a condensation product of naphthalene sulfonic acid and formaldehyde) as anionic dispersing agent of BASF. The dye was precipitated in a fine dispersion ready for use in dyeing after evaporation of the solvent by warming.
2.3.2. Dyeing of Polyester Fabrics
- The dye bath (1:20, good to dye liquor ratio) in a sealed stainless steel dye pots of 250 ml capacity in “Galvanin-Marino VI-Italy” dyeing machine. Additional dispersing agent (0.5-1.0 g/l) was added and the pH of the bath adjusted to 5.5 using glacial acetic acid. Dyeing carried out by raising the dye bath temperature from 20 to 130°C at a rate of 3 °C/min and holding at this temperature for 60 min before rapidly cooling to 50°C at 9.9°C/min. The dyed fabrics were then rinsed with cold water, reduction-cleared using sodium hydroxide (2 g/l) and sodium hydrosulphite (1 g/l) and soaped with 2% nonionic detergent and ammonia (pH 8.5) at 50°C for 30 min to improve washing fastness.
2.3.3. Color Fastness Tests
- The color fastness of dyeing was evaluated using the standard method[17] and given in Table 1. The fastness to light, sublimation and perspiration was assessed in accordance with AATCC-15 (1985). The rubbing fastness test was carried out with a crockmeter (Atlas) in accordance with AATCC-88 (1988) and the wash fastness test in accordance with IS: 765-1979.
2.3.4. Color Assessment
- The colorimetric parameters (Table 2) of the dyed polyester fabrics were determined on a reflectance spectrophotometer (Gretag Macbeth CE 7000a), equipped with a D65/108 source and barium sulphate as standard blank, UV excluded specular component included and three repeated measurements average settings.
3. Results and Discussions
3.1. Synthesis and Tautomeric Structure
- The synthetic approach to the target azo dyes is outlined in Scheme 1. The starting material, 4-hydroxycoumarin (3), was prepared by the Claisen condensation of 2-hydroxyacetophenone (1) with the diethylcarbonate (2) in the presence of sodium hydride as previously described[18]. The coupling of 3 with a variety of aromatic diazonium salts in an ethanolic sodium hydroxide solution at 0-5°C produced the respective of 3-aryl(hetaryl)hydrazono-2,4- chromandiones 4a-I (named as 2,3,4-chromantrione-3-aryl (hetaryl)hydrazones by the authors). In a similar manner coupling of each tetrazotized p-phenylenediamine and benzidine with two moles of 3 under the same conditions afforded the respective bishydrazono derivatives 4j and 4k (Scheme 1).
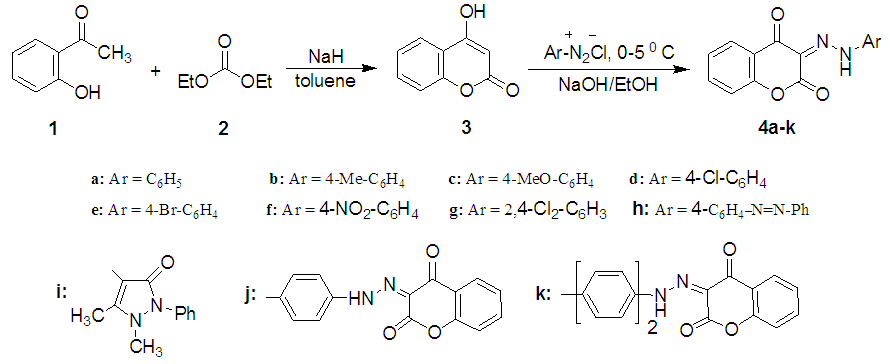 | Scheme 1. Synthesis of 3-aryl(hetary)hdrazono-2,4-chromndiones 4a-k |
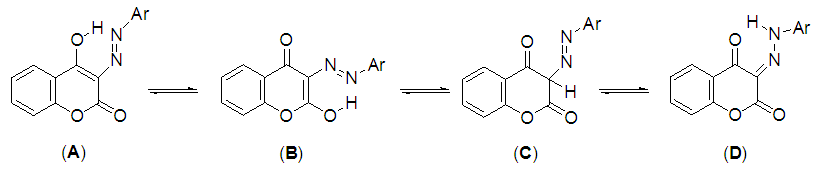 | Figure 1. Possible tautomeric structures of 3-arylhdrazono-2,4-chromandine(A-D) |
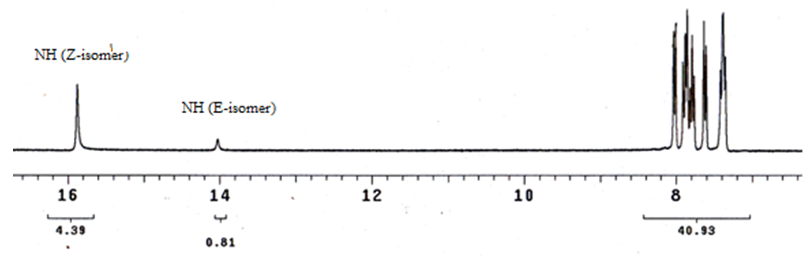 | Figure 2. Partial 1H NMR spectra of 4e in DMSO-d6 in which peaks are assigned to the presence of the two isomers of E and Z-isomer (see Fig. 3) |
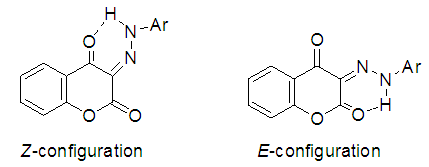 | Figure 3. Possibilities configuration for the compounds 4a-k as they exist in the tautomeric form(D) |
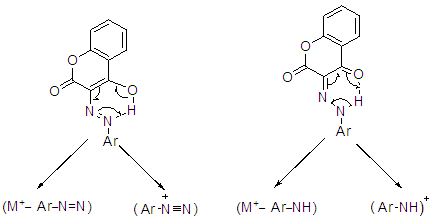 | Figure 4. Fragmentation pathway appeared for the hydrazone and azo moiety as tepresentative example |
3.2. Dyeing and Fastness Properties
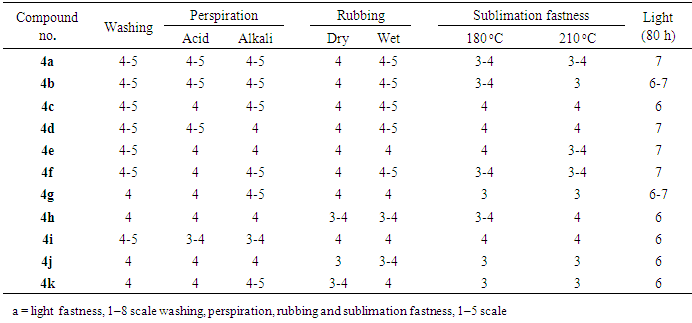
- The development of disperse dyes “Dyes insoluble in water and applied from aqueous dispersion rather than from solution” was a revolutionary solution to the problem of coloration of synthetic fibers. The dyes were essentially insoluble in water and were prepared for application by being ground, in the presence of dispersing agents, to microscopically fine particles of the order of a few microns and, then, by pan drying the resultant suspension. The resulting readily dispersible solid could then dye the more hydrophobic acetate fiber by partitioning into the fiber from low dye bath concentrations. The synthesized disperse dyes under investigation 4a-k were applied to polyester fabric at 2% shade by high-temperature pressure technique (130°C). The dyes on polyester fabrics were evaluated in terms of their fastness properties as shown in Table 1.
|
3.2.1. Fastness to Washing
- The dyed polyester fabrics have very good fastness to washing according to the international Geometric Grey scale[35] and this may be attributed to inadequate diffusion of dye molecule into the fabrics.
3.2.2. Fastness to Perspiration: (Acid and Alkaline)
- The high ratings for change in color at both acidic and alkaline conditions indicate that the sensitivity of the dyed samples are not related to PH. This may be due to the stability of the dyes towards degradation under either acidic or basic conditions.
3.2.3. Fastness to Rubbing
- The test is designed to determine the amount of color transferred from the surface of colored fabrics to another surface by rubbing. Most of the dyes have a good rubbing fastness and this may be attributed to adequate diffusion of dye molecule into the fabrics.
3.2.4. Fastness to Sublimation
- Sublimation fastness properties of synthesized dyes expressed as color staining on the undyed polyester piece ranged from moderate to good according to the international Geometric Grey Scale[35]. It is dependent upon the structure of the coupling component and was influenced by the substituents in the coumarin ring. Most of the dyes showed good sublimation fastness.
3.2.5. Fastness to Light
- It is significantly depended on nature of the substituents which change the electron density around hydrazo group. The high fastness to light may be attributed to electron accepting groups. This agrees with the notion that the azo compounds appended with electron-withdrawing substituents on the diazo components are less prone to photofading[36]. Generally, the prepared dyes showed satisfactory fastness to light ranging 6-7 according to the international Geometric Grey Scale[35].
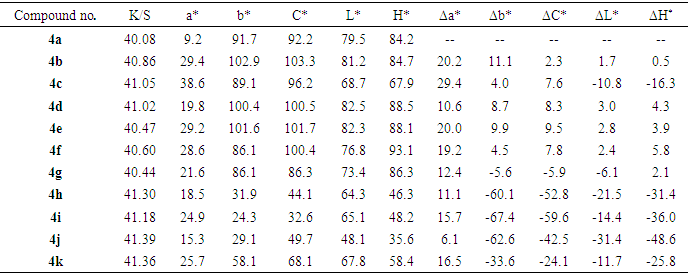
3.3. Color Assessment
- The color parameters (Table 2) of the dyed polyester fabrics were measured using a spectrophotometer (GretaMacbeth Color Eye 7000A, USA) under illuminant D65, with a 10° standard observer. The assessment of color-dyed fabrics was made in terms of tristimulus colorimetry. The following CIELAB coordinates are measured, lightness (L(*, chroma (C*), hue angle from 0o to 360° (h), (a*) value represents the degree of redness (positive) and greenness (negative) and (b*) represents the degree of yellowness )positive) and blueness (negative). The K/S value which has been employed as the dye uptake or color strength was calculated for each dyed specimen as well as for an undyed one at a maximum absorption wavelength of 360 nm from the reflectance values using the Kubelka-Munk equation[36] as followsK/S = (1-R)2 / 2Rwhere K is the coefficient of absorption S is the coefficient of scattering R is the reflectance value of the fabric at peak wavelength. The parent dyestuff in each group is taken as the standard in the color difference calculation (ΔL*, ΔC*, and ΔH*). The results were obtained using CIELAB techniques, and are given in Table 2, where ΔL* is the lightness difference, ΔC* is the chroma difference, and ΔH* is the hue difference. A negative sign of ΔL* indicates that the dyed fabric becomes darker than the standard, whereas a positive sign indicates that the dyed fabric becomes lighter than the standard. A negative sign of ΔC* indicates that the dyed fabric becomes duller than the standard, whereas a positive sign indicates that the dyed fabric becomes brighter than the standard. A negative sign of ΔH* indicates that the color shifted to a red color, while a positive sign indicates that the color shifted to yellowish. The results are shown in Table 2 tend to give the following conclusions:i. K/S values in the dyes under investigation 4a-k vary from 40.08 to 41.39, which all members of this groups increases strength of the K/S value of the polyester fabric compared with that of the parent 4a. Dyes 4h-k which contain bis-hydrazo or hetero moiety are characterized by higher K/S values compared with their analogues 4a-g, indicating that the introduction of bis-hydrazo or hetero moiety in dyes increases the color strength on polyester fabrics.ii. The color hues of the dyes under investigation 4a-k on polyester fabric are shifted to the yellowish direction on the yellow-blue axis according to the positive values of b*. iii. The color hues of the synthesized dyes 4a-k on polyester fabrics are shifted to the reddish direction on the red_green axis as according to the positive values of a* for these dyes.iv. In general, dyeings with the dyes 4g-k were darker and duller than the other dyes 4a-f according to the values of L* and C*.
|
4. Conclusions
- In conclusion, we described the synthesis and spectroscopic properties of a series of disperse azo dyes 4a-k including coumarin ring. The spectroscopic data of the dyes prepared has provided a decisive evidence that such compounds exist predominantly in the hydrazo structure (D) as a mixture of Z and E-isomer with ratio ~ 84.5:15.5. Finally the prepared dyestuffs were dyed on polyester fabrics and subsequently their dyeing properties, light, washing, perspiration, rubbing and sublimation fastnesses were determined. All these properties were proved to be good. From these investigations, it may be concluded that dyestuffs derived from 4-hydroxycoumarin are reasonable azo disperse dyestuffs giving good all round properties on polyester fabrics.
ACKNOWLEDGMENTS
- Financial support of this research through a grant from the Academy of Scientific Research and Technology, Egypt (to Moaz M. Abdou) is greatly appreciated. The authors express their appreciation and gratitude to the authorities of the Laboratories and Research sector in El-Nasr Company for Textile, Spinning and Weaving, El-Mahalla El-Kubra, Egypt for dyeing assessment, fastness tests and color measurements.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML