-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(6): 326-334
doi: 10.5923/j.chemistry.20120206.06
Bioaccumulation of Heavy Metals in Fishes of Hashenge Lake, Tigray, Northern Highlands of Ethiopia
Abraha Gebrekidan Asgedom1, 2, Mulu Berhe Desta1, Yirgaalem Weldegebriel Gebremedh3
1Department of Chemistry, College of Natural and Computational Sciences, Mekelle University, P.O.Box 231, Mekelle, Ethiopia
2Department of Chemical Engineering, Laboratory of Applied Physical Chemistry and Environmental Technology, Katholieke Universiteit Leuven, Belgium
3Ezana Analytical Laboratory in Ezana Mining Development PLC, Mekelle, Tigray, Ethiopia
Correspondence to: Abraha Gebrekidan Asgedom, Department of Chemistry, College of Natural and Computational Sciences, Mekelle University, P.O.Box 231, Mekelle, Ethiopia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
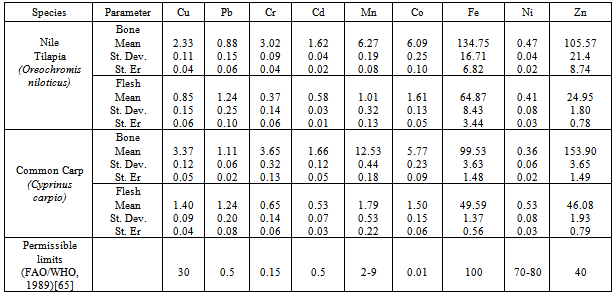
Water samples, bottom sediments, Oreochromis niloticus (Nile Tilapia), and Cyprinus carpio(Common Carp) from Lake Hashenge, Tigray, Northern Ethiopia were analysed quantitatively for the presence of copper, iron, zinc, cobalt, lead, nickel, chromium, manganese and cadmium using Atomic Absorption Spectrophotometer. The concentrations of 9 elements in Tilapia flesh, Tilapia bone, Common Carp flesh and Common carp bone (mg element/kg dry mass) were: Cu 0.85, 2.33, 1.40, 3.37; Pb 1.24, 0.88, 1.24, 1.11; Cr 0.37, 3.02, 0.65, 3.65; Cd 0.58, 1.62, 0.53, 1.66; Mn 1.01, 6.27, 1.79, 12.53; Co 1.61, 6.09, 1.50, 5.77; Fe 64.87, 134.75, 49.59, 99.53; Ni 0.41, 0.47, 0.36, 0.53 and Zn 24.95, 105.57, 46.08, 153.90, respectively. Studies on the different parts of the fish revealed higher concentrations of heavy metals on the bones than flesh parts of both the Nile Tilapia and Common Carp fishes. In most of the fish samples, lead, chromium, cadmium, cobalt and zinc concentration were found to be above the maximum tolerable values provided by FAO/WHO (1989). Results also revealed organ specific distribution of heavy metals in Common Carp and Nile Tilapia, which has been due to the physiological role in fish and/or the likely influence of natural and anthropogenic sources on Hashenge Lake.
Keywords: Heavy metals, Bioaccumulations, Nile Tilapia, Common Carp, Water pollution, Hashenge Lake
Cite this paper: Abraha Gebrekidan Asgedom, Mulu Berhe Desta, Yirgaalem Weldegebriel Gebremedh, "Bioaccumulation of Heavy Metals in Fishes of Hashenge Lake, Tigray, Northern Highlands of Ethiopia", American Journal of Chemistry, Vol. 2 No. 6, 2012, pp. 326-334. doi: 10.5923/j.chemistry.20120206.06.
Article Outline
1. Introduction
- Food safety is a major public health worldwide. During the last decades, the increasing demand of food safety has stimulated research regarding the risk associated with consumption of foodstuffs contaminated by pesticides, heavy metals and/or toxins[1]. During the last decades the rapid economic development of Africa has also led to an increase in environmental pollution[2],[3], and[4]. Heavy metals are among the major contaminants of food supply and may be considered as the most important problems to our environment[5],[6]. These problems are getting more serious all over the world especially in developing countries. Heavy metals, in general, are not biodegradable, have long biological half-lives and have the potential for accumulation in the different body organs leading to unwanted side effects[7],[8],[9],[10], and[11]. Most of the heavy metals are extremely toxic and readily reaches to toxic level[7],[12], and[13]. Food chain contamination is one of the important pathways for the entry of these toxic pollutants in to the human body[8],[9],[10],[14],[15],[16],[17],[18].Fish is one of the most important food sources of animal origin because of its higher nutritive values that contains high quality animal protein, lipids, vitamins, essential fatty acids and several kinds of minerals[19],[20],[21],[22] and an important source of income to many people in developing countries[23]. In Africa, some 35 million people depend wholly or partly on fishery for their livelihood[24]. Recently the consumption and demand for fish as a cheap source of protein is increasing in Ethiopia. The fish supply in most cases comes from the major lakes such as Fincha, Hawassa, Ziway, Koka, Abaya, Hashenge and rivers in the country[25]. The fish production from these water bodies is supporting the livelihood of poor farmers living around water bodies in providing inexpensive, but high quality protein and diversifying sources of income.Currently, the pollution of the aquatic environment with heavy metals has become a worldwide problem because they are indestructible, potential toxic effects on organisms[26], [27] and ability to bioaccumulate in aquatic ecosystems[28], [29],[30],[31]. Heavy metal concentrations in aquatic ecosystems are usually monitored by measuring their concentrations in water, sediments and biota[32], which generally exist in low levels in water and attain considerable concentration in sediments and biota[33]. Heavy metals including both essential and non-essential elements have a particular significance in ecotoxicology, since they are highly persistent and all have the potential to be toxic to living organisms[34].Studies on heavy metals in rivers, lakes, fish and sediments[35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45] have been a major environmental focus especially during the last decade. Sediments are important sinks for various pollutants like pesticides and heavy metals and also play a significant role in the remobilization of contaminants in aquatic systems under favourable conditions and in interactions between water and sediment. Therefore, heavy metals can be bioaccumulated and biomagnified via the food chain and finally assimilated by human consumers resulting in health risks[46]. As a consequence, fish are often used as indicators of heavy metals contamination in the aquatic ecosystem because they occupy high trophic levels and are important food source[39],[46],[47],[48],[49], and[50].Recently, assessment of heavy metals residues in fish and its effects on the health of people are attracting the interest of many researchers from different countries. Essential metals such as Cu, Zn and Fe have normal physiological regulatory functions, but may bioaccumulate and reach toxic levels[51]. Non-essential metals are usually potent toxins and their bioaccumulation in organisms lead to intoxication, decreased fertility, tissue damage and dysfunction of a variety of organs[40].Currently, Ethiopia has set no guideline values on the levels of heavy metals in fish resources. The purpose of this study was to produce baseline data on the distribution of heavy metals, such as Cu, Pb, Cr, Cd, Mn, Co, Fe, Ni and Zn in water, sediment and edible parts of commonly consumed fish tissues (flesh and bone) of Oreochromis niloticus (Nile Tilapia) and Cyprinus carpio (Common Carp) obtained from Hashenge Lake. In addition to providing information for background levels of metals, analysis of the enrichment of these heavy metals in water, sediment and fish samples was used to evaluate the magnitude, impacts and possible sources of heavy metal contamination on Hashenge Lake. The results obtained from this study would also provide base line information on the levels of heavy metals in water, sediment and fish of the lake, contributing to the effective monitoring of both environmental quality and the health of the organisms inhabiting Hashenge Lake. To the best of our knowledge, no work has been carried out on the extent of heavy metal concentration in fish samples of Hashenge Lake and their potential impacts on the food chain and human health risks.
2. Materials and Methods
2.1. Study Area
- Hashenge Lake (Figure 1), also called Lake Ashangi, is located in the coordinates of 12°34′50″N and 39°30′00″E in Tigray, Northern highlands of Ethiopia at an elevation of 2440 meters above sea level. It is one of the crater lakes in the country and not associated with the East African rift system; instead it is the result of volcanism. This lake has no any outlet to drain its water. Hashenge Lake is five kilometres long and four kilometres wide, with a surface area of 20 square kilometres. Its drainage area is 129 Km2 and has a maximum depth of 25 meters.
 | Figure 1. Map of study area |
2.2. Sampling
- Homogenized water samples were taken in cleaned 2 litre polyethen bottles from different sampling points at surface, middle and bottom of the lake using 3 L Heart Valve water sampler. The fish were sampled with gill nets from the lake. Adult fish of similar size were selected from both fish types and five fish samples of each type were taken for analysis. Sediment samples were collected from the lake using bottom sediment Grab Sampler. Water and fish samples were brought to the laboratory using cold box and stored in refrigerator until analysis.
2.3. Sample Preparation
- About 100 ml of the water samples were filtered through Nitrocellulose filter membrane of 0.45 μm pore size prior dried in 105℃ for 2 hours. The filtrate and unfiltered water samples were preserved in 2 ml concentrated nitric acid to prevent precipitation of metals and growth of algae. Dissolved metals were determined from the filtrate where as the total metals from the unfiltered water samples using nitric acid digestion[52][52]. Finally 20 mL of each of the filtered and digested samples were taken and analyzed for trace metals using Varian AA240 FS Atomic Absorption Spectrometer.Soil samples taken from the lake were air-dried, mixed, quartered and one fourth of each sample was dried in an oven at 105℃ for 12 h. The dried samples were then ground and sieved with 75 μm mesh size. A 20 + 0.05 g of pulverized (75 μm) sample was weighed into a 400 ml tall form beaker. An acid mix of 50 ml HCl and 20 ml HNO3 was slowly added to the sample while swirling, to ensure the sample is properly wetted and simmered on the hot plate for a minimum of 45 minutes at 160℃, stirring with a glass rod. It was removed from the hot plate before dryness, cooled and diluted on a 200 ml volumetric flask with distilled water, shaken and poured back into the beaker and settled for 30 minutes. Finally 20 mL was taken by 16x150 mm test tubes and analyzed for trace metals using Varian AA240 FS Atomic Absorption Spectrometer.Fish, Oreochromis niloticus (Nile Tilapia) and Cyprinus carpio (Common Carp), were washed to remove slime and/or ice drained and water was removed with tissue paper. The samples were dried in an oven at an initial temperature of 70 ℃ for 3 hrs and increased temperature to about 105℃ for 12 hours. After allowing to cool overnight, samples were further heated for 8 hrs at 105℃. Then, samples were cooled and exposed to ambient laboratory temperature to air-dry for three days. Finally, flesh and bone parts of the whole fish were separated and ground into powder using a porcelain mortar and pestle. A sample of 2.5 g was placed into Teflon beaker and 20 ml of mixed concentrated HNO3-H2O2 (3:1) was added. The digestion system was swirl to ensure a proper dispersion and left for 48 hrs at room temperature. After refluxing the mixture on a heating mantle until fume ceases, 5 ml of HClO4 was added and further refluxed for 30 min. Then the digest was cooled and finally diluted to 100 ml with deionised water. The sample solution was clear. A blank digest was carried out in the same way. All metals were determined against aqueous standards.
2.4. Sample Analysis
- Fully automated PC-controlled true double-beam Atomic Absorption spectrometer with Fast Sequential operation (Varian AA240 FS) for fast multi-element flame AA determinations with features 4 lamp positions and automatic lamp selection was used for the determination of metals (Cu, Pb, Cr, Cd, Mn, Co, Fe, Ni and Zn) at Ezana Analytical Laboratory, Ezana Mining PLC, Mekelle, Tigray, Ethiopia, with instrument working condition shown in Table 1. The spectrometer was operated with SpectrAA Base and PRO software. The descriptive statistical anlysis (mean, standard deviation and standard error) were conducted using the Excel software.
2.5. Working Solutions
- Stock standard solutions containing 1000 mgL-1 of Cu, Pb, Cr, Cd, Mn, Co, Fe, Ni and Zn (SPECTROSCAN, Industrial Analytical (ptv) Ltd, South Africa) were used for preparing working standards (0.05, 0.10, 0.50, 1.00 mgL-1). Calibration curves were prepared separately for all the metals and analysed using the working standards. The instrument was set to zero concentration for all types of samples, using a reagent blank. Digested samples were aspirated into the fuel-rich air-acetylene flame and the metal concentrations were determined from the calibration curves. Each determination was based on the average values of three replicate samples. The precision of the analytical procedures, expressed as relative standard deviation, ranged from 5 to 10%.
2.6. Bioavailability
- Bioavailability is the amount of heavy metals in a water-soluble form that can plants and animals readily uptake and assimilate[53]. The bioavailability of metals (expressed in percent) with respect to total metal content can be calculated as follows:
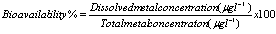 Where the dissolved metal concentration is determined via analysis from filtered water samples and the total metal concentration from unfiltered water samples.
Where the dissolved metal concentration is determined via analysis from filtered water samples and the total metal concentration from unfiltered water samples. 2.7. Bioaccumulation Factor
- The bioaccumulation of the heavy metals (HM) in the samples of the lake was quantified with a bio-accumulation factor (BAF), defined as the ratio of the concentration of a specific heavy metal in the organism (muscle and bone of the fish) to the concentration of the metal in the Lake water[54]. The BAF was calculated as follows:
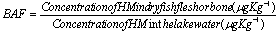
3. Results and Discussions
3.1. Heavy Metal Contamination in Water and Sediments
- The concentrations of heavy metals in water from the Hashenge Lake are presented in Table 2. Heavy metals were found to be in the following increasing order of concentrations (µg L-1): Zn (937.5) > Mn (20) > Cd (8.7) > Fe (3.6) > Co (3.5) > Cr (3.4) > Pb (3.3) > Ni (2.3) > Cu (2.1). The concentration of zinc in the water sample constituted the major portion of the total metal ions determined (95.24%), while Cu concentration was the lowest (0.21%). The average concentration of Cd, in the water samples exceeded the permissible limits prescribed by WHO[55] and USEPA[56]. The average concentrations of heavy metals in sediments of the Lake are presented in Table 3. In the sediment sample the heavy metals were found to be in the following increasing order of concentrations (µg L-1): Ni (39423.5) > Zn (1129) > Cd (206) > Cr (86.5) > Mn (71) > Cu (56) > Co (34) > Pb (3) > Fe (2). The Ni concentration in the sediment sample constituted the major portion of the total metal ions determined (96.13%), while Fe concentration was the lowest (0.005%). Trace metals are considered to be major toxicant in contaminated water worldwide[57],[58],[59],[60] and studies indicated that levels of metals were higher in sediment than in water due to the adsorption of cations by organic matter present in the sediment layers. The metals interact with organic matter in aqueous phase and settle down resulting in high concentrations in sediments. Our studies also revealed that the comparisons of the average concentration of heavy metals in sediments of the lake are higher than their soluble concentrations in water. The bioavailability of heavy metals is shown in Table 4. The percentage bioavailability of the heavy metals exhibited a maximum and minimum values for Zn (91.64%) and Mn (8.44%), while Pb (80%), Cu (62.68%), Cr (60.72%), Ni (60%), Cd (45.34%), Co (26.17%), and Fe (15.77%), respectively. The difference in percent bioavailability of the examined heavy metals could be due to in part on the concentration of anions, chelating agents in the water, pH and red-ox status[61] of the lake.
|
|
|
3.2. Heavy metal concentration in Nile Tilapia and Common Carp fishes
- Concentration of nine metals in the bone and flesh of two fish species (Nile Tilapia and Common Carp) from the Hashenge Lake are shown in Table 5. In line with other studies[62],[63] our findings showed that heavy metals are accumulated in the bone and flesh of the Nile Tilapia and Common Carp fishes. The concentration of metals in water, sediment and different parts of the fishes indicate that there is an interrelation of metal accumulation in the various components of the fish as suggested by Farag et al.,[64]. The fish acquires metals both directly from water and sediment and indirectly through the food chain[64]. Metal concentrations of bone of the examined species were generally higher than those in flesh. Iron was the highest in both bone and flesh of Nile Tilapia while zinc and iron were highest in the bone and flesh of Common Carp fish, respectively.Figure 2 shows the accumulation of heavy metals in the bone and flesh samples of Nile Tilapia in the order of Fe > Zn>Mn > Co > Cr > Cu > Cd > Pb > Ni and Fe > Zn > Co > Pb > Mn > Cu > Cd > Ni > Cr, respectively with highest accumulation of Fe (51.08%) for bone and (68%) for flesh followed by Zn at 40.59% for bone and 26% for flesh. Similarly, the concentration of the heavy metals in the bone and flesh samples of Common Carp in the order of Zn > Fe > Mn > Co > Cr > Cu > Cd > Pb > Ni and Fe > Zn > Mn > Co > Cu>Pb>Cr>Cd = Ni, respectively with highest accumulation of Zn (55%) followed by Fe at 35% for bone and Fe (48%) followed by Zn at 45% for flesh.The concentrations of heavy metals determined in the bone tissues of the two fish species were sufficiently above the concentrations in the flesh. Compared to report from Aweke and Taddese[66] the concentrations of Cu, Pb, Cd, Mn, Co, and Ni were very low in both Nile Tilapia and Common Carp of the Hashenge Lake than fishes from Lake Awassa and Ziway. But a high concentration of Fe and Zn were observed in fishes of Hashenge Lake than fishes from Lake Awassa and Ziway[66].
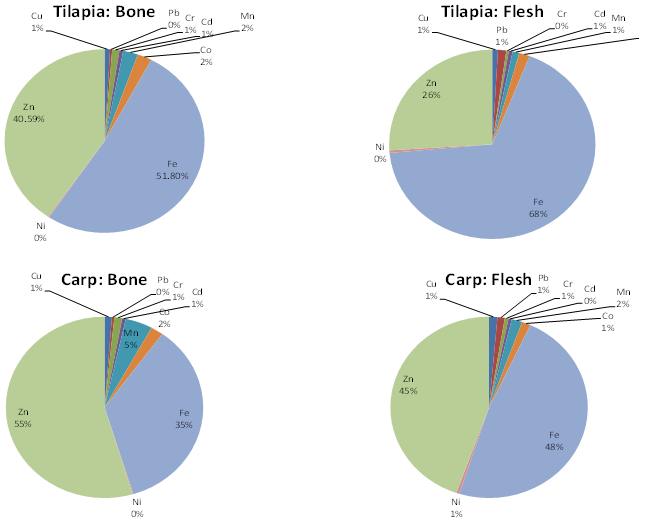 | Figure 2. Heavy metal content in fish samples of Nile Tilapia and Common Carp from Hashenge Lake |
|
|
4. Conclusions
- Although there is a gradual increase in production and consumption of fish in the regions, citizens have the right to get safe fish food which must be ensured that the fish are not contaminated beyond the acceptable safe limits. Toxic heavy metals enter into aquatic systems from various sources of polluted ecosystems. Most of the heavy metals discussed have toxic potential and their impact becomes apparent only after a long times of exposure. The results of this study indicated that there is a high accumulation of (above the permissible limits issued by FAO/WHO, 1989) Pb, Cr, Cd, Co and Zn in the flesh and bone of the fishes. Based on this, it is concluded that non-point sources lead to the contamination of the Hashenge Lake. It is, therefore, suggested that regular biomonitoring of heavy metal contaminants in fish is essential in order to prevent excessive build up of these toxic heavy metals in the human food chain. The quantities in fish flesh and bone measured in this study provide baseline information on concentrations and distribution of heavy metals in Nile Tilapia and Common Carp from Hashenge Lake, Tigray, Northern highlands of Ethiopia. The existence of pollution of both natural and anthropogenic origins, mainly agricultural practices, could be revealed through comprehensive investigations of other fish organs. Therefore, future studies should consider anthropogenic sources that contributed to differences in levels of heavy metals in the tissues of fishes obtained from Hashenge Lake and Ethiopia should set guideline values on the levels of toxic heavy metal contaminants in fish resources.
ACKNOWLEDGMENTS
- This work was done with the financial support from Mekelle University, Ethiopia. So, the authors gratefully acknowledge Mekelle University for the fund and those people involved in sample collection, preparation and analysis.
References
| [1] | D’Mello, J.P.F. Food safety: Contaminants and Toxins. CABI Publishing, Wallingford, Oxon, UK, Cambridge, MA, p. 480, 2003. |
| [2] | Ololade, I. A. and Oginni, O. “Toxic stress and hematological effects of nickel on African catfish, Clarias gariepinus, fingerlings”, Journal of Environmental Chemistry and Ecotoxicology Vol. 2(2) pp. 014-019. 2010. |
| [3] | Akiwumi, F.A and Butler, D.R. “Mining and environmental change in Sierra Leone, West Africa: a remote sensing and hydrogeomorphological study”, Environ Monit Assess 142,309–318, 2008. |
| [4] | Ochieng, E. Z., Lalah, J. O., Wandiga, S. O. “Analysis of Heavy Metals in Water and Surface Sediment in Five Rift Valley Lakes in Kenya for Assessment of Recent Increase in Anthropogenic Activities”, Bull Environ Contam Toxicol, 79:570-576, 2007. |
| [5] | Zaidi, M.I., Asrar, A., Mansoor, A., Farooqui, M.A. “The heavy metal concentrations along roadside trees of Quetta and its effects on public health”, J. Appl. Sci. 5 (4), 708–711, 2005. |
| [6] | Reilly, A., Lima DosSantos, C. and Michael Phillips “Food safety and products from aquaculture”, The FAO Aquaculture Newsletter, No. 19, 1998. |
| [7] | Kamaruzzaman, B.Y., Rina, Z., Akbar John, B. and Jalal, K.C.A. “Heavy metal accumulation in commercially important fishes of South West Malaysian coast”, Research Journal of Environmental Sciences, 1-8. 2011. |
| [8] | Kazim Uysal, Yılmaz Emre, Esengül Köse “The determination of heavy metal accumulation ratios in muscle, skin and gills of some migratory fish species by inductively coupled plasma- optical emission spectrometry (ICP-OES) in Beymelek Lagoon (Antalya/Turkey)”, Microchemical Journal 90, 67–70. |
| [9] | Jarup, L. (2003). Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182, 2008. |
| [10] | Senarathne, P and Pathiratne, K.A.S. “Accumulation of heavy metals in a food fish, Mystus gulio inhabiting Bolgoda Lake, Sri Lanka”, Sri Lanka J. Aquat. Sci. 12,61-75, 2007. |
| [11] | Barbara Jezierska and Malgorzata Witeska “The metal uptake and accumulation in fish living in polluted waters”, Soiland Water Pollution Monitoring, Protection and Remediation, 3–23, pp. 107-114, 2006. |
| [12] | Ei-lchiro Ochiai “Toxicity of Heavy Metals and Biological Defense: Principles and Applications in Bioinorganic Chemistry-VII”, Journal of Chemical Education,Volume 72 Number 6, pp. 479-484, 1995. |
| [13] | Mohammed K. Alam & Maughan O. Eugene “Acute toxicity of heavy metals to common carp (Cyprinus carpio”,. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, Volume 30, Issue 8, pp. 1807-1816, 1995. |
| [14] | Soisungwan Satarug, Scott H. Garrett, Mary Ann Sens, and Donald A. Sens “Cadmium, Environmental Exposure, and Health Outcomes, Review”, Environmental Health Perspectives, volume 118, number 2, pp. 182-190, 2010. |
| [15] | Bernier, J., Brousseau, P., Krzystyniak, K., Tryphonas, H. and Fournier, M. “Immunotoxicity of Heavy Metals in Relation to Great Lakes”, Environmental Health Perspectives, Vol 03, Supplement 9, pp.23-34, 1995. |
| [16] | Cheung, K.C. and Wong, M.H. “Risk assessment of heavy metal contamination in shrimp farming in Mai Po Nature Reserve, Hong Kong”, Environmental Geochemistry and Health, vol. 28, no. 1-2, p. 27-36, 2006. |
| [17] | Ferner, D. J. “Toxicity heavy metals”, eMed. Jor. 2 (5): 1, 2001. |
| [18] | Monica Harmanescu, Liana Maria Alda, Despina Maria Bordean, Ioan Gogoasa and Iosif Gergen “Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania”, Chemistry Central Journal, 5:64, 2011. |
| [19] | Aziz A. Fallah, S. Siavash Saei-Dehkordi, Amin Nematollahi, Tina Jafari “Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique”, Microchemical Journal 98, 275–279, 2011. |
| [20] | Gogus, U. and Smith, C. “n−3 Omega fatty acids: a review of current knowledge”, Int. J. Food Sci. Technol. 45 (2010) 417–436, 2010. |
| [21] | El-Kattan, Y.A. and Nahla, A. Abo-El Roos “Levels of some heavy metals in River Nile water and Oreochromis niloticus fish at Menoufia Governorate. Egypt”, J. Comp. Path. & Clinic. Path. Vol. 21 No. 1, 2008; 64- 75, 2008. |
| [22] | Darwish, A.M.; El- Mossalami, M.K. and El- Bassiony, R.A. "Quality assurance of some fatty fishes." Assuit Vet. Med. J., 49 (98): 79- 96, 2003. |
| [23] | FAO, State of world aquaculture. FAO Fisheries Technical Paper 500, Rome, Italy. 2006. |
| [24] | FAO, Fisheries and food security, Food for all, World food summit, Food and Agriculture Organization of the United Nations Viale delle Terme di Caracalla, 00100 Rome, Italy. 1996a. |
| [25] | FAO. Fisheries and Aquaculture topics. Quality and safety of fish and fish products. Topics Fact Sheets. In: FAO Fisheries and Aquaculture Department, Rome. 2003-2012. |
| [26] | Malik N., Biswas A.K., Qureshi T.A., Borana K., Virha R. “Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal”, Environ. Monit. Assess. 160: 267-267, 2010. |
| [27] | Macfarlane, G.B. and Burchett, M.D. “Cellular distribution of Cu, Pb and Zn in the Grey Mangroove Avicemmia marina (Forsk)”, Vierh Aquatic Botanic, 68: 45 – 49, 2000. |
| [28] | Javed, M. and Usmani, N. “Accumulation of heavy metals in fishes: A human health concern”, International Journal of Environmental Sciences, Volume 2, No 2, pp.659-670, 2011. |
| [29] | Ekeanyanwu, C.R., Ogbuinyi, C.A, and Etienajirhevwe, O.F. “Trace Metals Distribution in Fish Tissues, Bottom Sediments and Water from Okumeshi River in Delta State, Nigeria”. Ethiopian Journal of Environmental Studies and Management Vol.3 No.3, pp. 12-17, 2010. |
| [30] | Yousafzai, A.M. and Shakoori, A.R. “Heavy Metal Accumulation in the Gills of an Endangered South Asian Freshwater Fish as an Indicator of Aquatic Pollution”, Pakistan J. Zool., vol. 40(6), pp. 423-430, 2008 . |
| [31] | Censi, P., Spoto, S.E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., Di Geronimo, S.I., Punturo, R. and Ottonello, D. “Heavy metals in coastal water system. A case study from the north western Gulf of Thailand”, Chemosphere, 64: 1167 – 1176, 2006. |
| [32] | Camusso, M., Vigano, L. and Baistrini, R. “Bioaccumulation of trace metals in rainbow trout”, Ecotoxicology and Environmental Safety, 31: 133 – 141, 1995. |
| [33] | Namminga, H.N. and Wilhm, J. “Effect of high discharge and an oil refinery cleanup operation on heavy metals in water and sediments in skeleton creek”, Proceedings of the Oklahoma Academy of Science, 56: 133 – 138, 1976. |
| [34] | Storelli, M.M., Storelli, A., D’ddaabbo, R., Morano, C., Bruno, R and Marcotrigiano, G.O. “Trace Elements in loggerhead turtles (Caretta caretta) from the Eastern Mediterranean; Overview and Evaluation”, Environmental Pollution, 135: 163 – 170, 2005. |
| [35] | Abdel-Baki, A. S., Dkhil, M. A. and Al-Quraishy, S. “Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia”, African Journal of Biotechnology Vol. 10(13), pp. 2541-2547, 2011. |
| [36] | Shouta M. M. Nakayama, Yoshinori Ikenaka, Kaampwe Muzandu, Kennedy Choongo, Balazs Oroszlany, Hiroki Teraoka, Naoharu Mizuno, Mayumi Ishizuka “Heavy Metal Accumulation in Lake Sediments, Fish (Oreochromis niloticus and Serranochromis thumbergi), and Crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia, Arch”, Environ Contam Toxicol, 59:291–300, 2010. |
| [37] | Zahra Khoshnood, Amin Mokhlesi and Reza Khoshnood “Bioaccumulation of some heavy metals and histopathological alterations in liver of Euryglossa orientalis and Psettodes erumei along North Coast of the Persian Gulf”, African Journal of Biotechnology Vol. 9(41), pp. 6966-6972, 2010. |
| [38] | Ozmen, H., Kulahci, F., Cukurovali, A. and Dogru, M. “Concentrations of heavy metals and radioactivity in surface water and sediment of Hazar Lake (Elazig, Turkey)”, Chemosphere, 55: 401 – 408, 2004. |
| [39] | Begum, A., Amin, M.N., Kaneco S., Ohta K. “Selected elemental composition of fish, Tilapia nilotica, Cirrhina mrigala and Clarius batrachus from the fresh water Dhanmondi Lake in Bangladesh”, Food Chem 93:439 – 443, 2005. |
| [40] | Fernandes C., Fontainhas-Fernandes A., Cabral, D., Salgado M.A. “Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-paramos Lagoon, Portugal”, Environ. Monit. Assess 136: 267 – 275, 2008. |
| [41] | Pote, J., Haller, L., Loizeau, J.L Bravo, A.G., Satre, G. and Wildi, W. “Effects of sewage treatment plant oulet pipe extension on the distribution of contaminants in the sediments of the Bay of Vidy, lake Geneva, Switzerland”, Bioresource Technology. 99: 7122 – 7131, 2008. |
| [42] | Praveena, S.M., Radojevic, M., Abdullah, M.H. and Aris, A.Z. “Application of sediment quality guidelines in the assessment of mangrove surface sediment in Mengkabong lagoon, Sabah, Malaysia”, Iran J Environ Health Sci Eng5(1): 35 – 42, 2008. |
| [43] | Vinodhini R. and Narayanan, M. “Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp)”, Int. J. Environ. Sci. Tech. 5(2): 179-182, 2008. |
| [44] | Zafer Ayas, Guler Ekmekci, Sedat Vahdet Yerli and Murat Ozmen “Heavy metal accumulation in water, sediments and fishes of Nallihan Bird Paradise, Turkey”, Journal of Environmental Biology, 28(3) 545-549, 2007. |
| [45] | Ozturk, M., Ozozen, G., Minareci, O and Minareci, E. “Determination of heavy metals in issues of fishes, water and sediment from the Demirkopru Dam Lake (Turkey)”, Journal of Applied Biological Sciences, 2(3): 99 – 104, 2008. |
| [46] | Agah, H., Leermakers, M., Elskens, M., Fatemi, S.M.R., Baeyens, W. “Accumulation of trace metals in the muscles and liver tissues of five fish species from the Persian Gulf”, Environ. Monit. Assess. 157: 499- 514, 2009. |
| [47] | Özmen, H., Külahçı, F., Çukurovalı, A., and Doğru, M. “Concentrations of heavy metal and radioactivity in surface water and sediment of Hazar lake (Elazığ, Turkey)”, Chemosphere, 55: 401–408, 2004. |
| [48] | Svobodova Z., Celechovska O., Kolara J., Randak T., Zlabek V. “Assessment of metal contamination in the upper reaches of the Ticha Orlice River, Czech”, J. Anim. Sci., 49: 458-64, 2004. |
| [49] | Blasco, J., Rubio, J.A., Forja, J., Gomez-Parra, A., Establier, R. “Heavy metals in some fishes of the muglidae family from salt-pounds of Codiz Bay SW Spain”, Ecotox. Environ. Res. 1: 71-77, 1998. |
| [50] | Rashed, M. N. “Monitoring of environmental heavy metals in fish from Nasser Lake”, Environ Inter 27:27–33, 2001. |
| [51] | Tüzen, M. “Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry”, Food Chemistry, 80: 119-123, 2003. |
| [52] | SMEWW, APHA, Standard methods for the examination of water and wastewater, American Public health Association, (17 Edition), pp. 3-1 to 3-17, 1989. |
| [53] | Klavins, M., Briede, A., Parele, E., Rodinov, V., Klavina, I. “Metal accumulation in sediments and benthic invertebrates in Lakes of Latvia”, Chemosphere 36(15):3043–3053, 1998. |
| [54] | WHO. Guidelines for drinking water quality, World Health Organization, Geneva, 2008. |
| [55] | U.S. Environmental Protection Agency. Regional Screening levels (RSL) for Chemical Contaminants at Superfund Site, 2008. |
| [56] | Chi-Man, L. and Jiu, J. J. “Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in Mid-Levels area, Hong Kong”, Water Research, 40(4): 753-767, 2006. |
| [57] | Katsoyiannis, I. A. and Katsoyiannis, A. A. “Arsenic and other metal contamination of ground waters in the industrial area of Thessaloniki, Northern Greece”, Environ. Monit. Assess, 123: 1-3, 2006. |
| [58] | Asonye, C. C., Okolie, N. P., Okenwa, E. E. and Iwuanyanwu, U. G. “Some physico-chemical characteristics and heavy metal profiles of Nigerian rivers, streams and waterways”, African Journal of Biotechnology, 6(5), 617-624, 2007. |
| [59] | Yasuhiro, S., Duong, V. T., Daigo, S., Doan, C. and Yoshito, K. “Arsenic and other metal contamination of groundwater in the Mekong River Delta, Vietnam”, Journal of Health Science, 53(3): 344-346, 2007. |
| [60] | Nayaka, B. M. S., Ramakrishna, S., Jayaprakash and Delvi, M. R. “Impact of heavy metals on water, fish (Cyprinus cerpio) and sediment from a water tank at Tumkur, India”, Int. J. Ocen. Hydrobiol., 38(2): 17-28, 2009. |
| [61] | Cross, F.A., Hardy, L.H., Jones, N.Y., Barber, R.T. “Relation between total body weight and concentration of Mn, Fe, Zn, Cu and Hg in the blue fish (Pomatonus saltatrix) and a bathy dermersal fish (Antimora rostrata)”, J. Fish. Res. Bd. Can. 30: 1287-1291, 1973. |
| [62] | Eustace, I. J. “Zinc, cadmium, copper and manganese in species of finfish and shellfish caught in the Derwent Estuary, Tasmania”, Aust. J. Mar. Fresh. Res. 25: 209-220, 1974. |
| [63] | Farag, A. M., Nimick, D. A., Kimball, B. A., Church, S. E., Harper, D. D. and Brumbaugh,W. G. “Concentrations of metals in water, sediment, biofilm, benthic macroinvertebrates, and fish in the Boulder River watershed, Montana, and the role of colloids in metal uptake”, Arch. Environ. Contam. Toxicol., 52: 397–409, 2007. |
| [64] | WHO/FAO. National Research Council Recommended Dietary 626 Allowances (10th ed). National Academy Press. Washington, DC. USA. 1989. |
| [65] | Aweke Kebede and Taddese Wondimu “Distribution of trace elements in muscle and organs of tilapia, oreochromis niloticus, from Lakes Awassa and Ziway, Ethiopia”, Bull. Chem. Soc. Ethiop., 18(2), 119-130, 2004. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML