-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(6): 322-325
doi: 10.5923/j.chemistry.20120206.05
Assessment of the Value of the Antacid Contents of Selected Palestinian Plants
Orwa Jaber Houshia1, Mohamad AbuEid2, Oday Zaid2, Motasem Zaid2, Najat Al-daqqa1
1Department of Chemistry, Arab American University, P.O. Box 240, Jenin, Palestine
2Department of Natural Resources, National Agricultural Research Center, Jenin, Palestine
Correspondence to: Orwa Jaber Houshia, Department of Chemistry, Arab American University, P.O. Box 240, Jenin, Palestine.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A variety of herbal plants have been used worldwide to remedy various diseases. The healing aspect of herbal plants have been accepted in many cultures and have been known as alternative medicine. Some herbs may consist of pharmaceutical ingredients suitable to treat certain cases such as stomach acidity or ulcers. Our aim was to verify the claim that some herbal “folk” plants can be used as an alternative for neutralizing the stomach acidity. Therefore, the purpose of this research was to test selected herbal plants for anti acid efficacy and estimate the acid-neutralizing capacity by addition of excess acid, followed by back-titration of the excess acid with sodium hydroxide. The anti acid capacity of the herbs was compared with that of anti acid tablets containing magnesium hydroxide and calcium carbonate as the active ingredient.
Keywords: Herbal Plants, Antacid Capacity, Back-Titration, Anti-Ulcer Medicine
Cite this paper: Orwa Jaber Houshia, Mohamad AbuEid, Oday Zaid, Motasem Zaid, Najat Al-daqqa, "Assessment of the Value of the Antacid Contents of Selected Palestinian Plants", American Journal of Chemistry, Vol. 2 No. 6, 2012, pp. 322-325. doi: 10.5923/j.chemistry.20120206.05.
Article Outline
1. Introduction
- Excessive secretion of gastric acid or stomach acid, which is mostly hydrochloric acid (HCl), inflames the stomach lining and produces ulceration. Some common causes might be an infection with a bacterium called Helicobacter pylori, or long-term use of non-steroidal anti-inflammatory medicines (NSAIDs) such as aspirin and ibuprofen, and other factors that make heartburn worse such as stress and spicy foods[1]. Antacids relieve the effects of the ulcer by neutralizing small amounts of excess stomach acid. Several antacid tablets are available over the counter and sold under various commercial names such as Maalox, Mylanta, Rolaids, Tums, Gaviscon, Alka-Seltzer, and Rennie, among others[2]. The main common active ingredients in these tablet consists of one or combination of magnesium hydroxide, aluminum hydroxide, calcium carbonates, magnesium carbonate, and Sodium bicarbonate[3]. Alternative to such tablets is the use of natural plant extracts to combat stomach acidity in what has become known as “folk” or traditional medicine[6]. Recent literature indicated that extracts from herbal plants are being used worldwide for treatment of peptic ulcer[4-7]. The market of herbal plant products, which contain flavonoids, terpenoids and tannins as the active substances, has flourished significantly[8]. In Palestine, many people, especially the elderly, use alternative medicine to remedy various diseases[9]. These traditional herbal remedies have been passed on and used from generation to generation based on personal experience rather than scientific experiments. The aim of this project is to experimentally determine the antacid capacity of some of these herbal medicines to verify the claim. Quantitative determination will be carried out by titration with standardized sodium hydroxide solution. In aqueous solutions, a the acidic H+ions the herbs may produce will be titrated with OH–ions from the standardized NaOH base. The reaction of an acid and base is a neutralization reaction, the products of which are a salt and water. In an aqueous solution, virtually all of the OH– ions present will react with all of the H+ ions which are present:
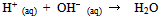 | (1) |
2. Experimental
2.1. Standardization of a Sodium Hydroxide Solution
- Because sodium hydroxide is hygroscopic (absorbs water readily from air), it was standardized using potassium acid phthalate, KHPh, primary standard. In our experiments, a solution of NaOH, which has an approximate concentration of 1.0 M, was standardized using potassium acid phthalate, KHPh . The molecular weight of KHPh is 204.23 g/mole, and it has one acidic proton, which will react quantitatively with OH–: OH– (aq) + KHPh (aq) → H2O (l) + KPh– (aq)For the highest accuracy, a sample size is chosen such that it will consume as large a volume of the base as possible without exceeding the capacity of the buret. The 50 mL buret was used, and the amount of KHPh was chosen such that it required approximately 20 mL of 1.14 M NaOH solution to reach the endpoint. Thus, about 0.0228 moles, or 4.656 g, of KHPh was weighed and used. At the endpoint, the number of moles of NaOH equals the number of moles of KHPh used: Moles NaOH = moles KHPh / Volume NaOH in liters or Moles NaOH = (g, KHPh) / (204.23 g/mole) x (1000 mL/L )/(mL, NaOH ) Once the NaOH solution was standardized, it was used for titrating the herbal plants.
2.2. Sample Preparation
- About 1.0 gram of each sample was crushed using a mortar and pestle. The crushed samples were weighed to the nearest 0.001 g as shown in the tables, and transferred to a 250-mL Erlenmeyer flask. Exactly 25 mL of 0.521 M HCl was added to each of the flasks and gently swirled. The flasks were heated gently to boil for exactly 5 minutes to remove any interference from the dissolved carbonic acid that may have come from the CO2 air and dissolved in water. The CO2 was driven off by heating the contents of the flask just to boiling:
 | (1) |
3. Results and Discussion
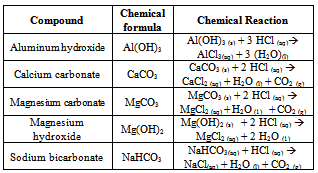
- Antacids relieve the effects of heartburn by neutralizing small amounts of excess stomach acid. Stomach acid is mostly hydrochloric acid (HCl). The active ingredients in commercial antacids are usually either insoluble metal carbonates, such as calcium carbonate (CaCO3), or insoluble metal hydroxides, such as magnesium hydroxide, Mg(OH)2. These substances are mixed with a variety of inert materials that serve as binders. Table 1 shows some the most common active ingredients in these tablet. These compounds neutralize stomach acid to produce salts and water.
|
|
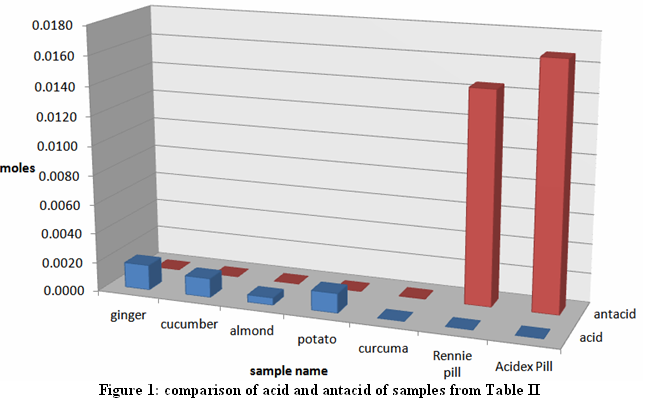 | Figure 1. Comparison of acid and antacid of samples from Tabl |
4. Conclusions
- Patients with excessive secretion of gastric acid should consult medical doctors before taking any herbal treatments. As shown from the experiments, some of these herbal remedies may not contain the antacid outcome sought. With the tools and techniques of back-titration, the research suggests that the herbal plants in this study contained zero to little antacid fighting power compared with commercially available tablets from chemistry point of view. Further investigation from a biological and physiological perspectives would be also valuable.
ACKNOWLEDGEMENTS
- Appreciation is expressed to the staff of the Natural Resources Department at NARC for their help, and Ms. Neebal Omar Zain at AAUJ chemistry department for her help.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML