-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(6): 299-305
doi: 10.5923/j.chemistry.20120206.02
A Study of Stability Constants of [Zn – L-Amino Acidate – Vitamin-PP] Systems
Kavita Rai, Farid Khan
Electrochemical Laboratory, Department of Chemistry, Dr. H.S. Gour University, Sagar, 470 003, Madhya Pradesh, India
Correspondence to: Farid Khan, Electrochemical Laboratory, Department of Chemistry, Dr. H.S. Gour University, Sagar, 470 003, Madhya Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The polarographic technique was used in formation of zinc (II) mixed ligand complexes of amino acids viz. glycine, α-alanine, L-leucine, L-valine, L-asparagine L-glutamine used as primary ligands and vitamin-PP (nicotinamide) as the secondary ligand at pH = 7.30 ± 0.01, µ = 1.0 M NaClO4 at 25℃ and 35℃. The complexes with metal to ligands ratio 1:1: 1, 1: 1:2 and 1: 2: 1 have been reported. The stability constants varied from 1.91 to 11.50. The kinetic parameters and thermodynamic parameters were also also determined. The values of standard rate constant (k) were of the order of 10-3cm sec-1 confirmed the quasireversible nature of electrode processes. The thermodynamic parameters reveal that complexes were lesser stable at high temperature.
Keywords: L-amino Acids, Zinc (II), Vitamin-PP, Stability Constants, Kinetics, Thermodynmics
Cite this paper: Kavita Rai, Farid Khan, "A Study of Stability Constants of [Zn – L-Amino Acidate – Vitamin-PP] Systems", American Journal of Chemistry, Vol. 2 No. 6, 2012, pp. 299-305. doi: 10.5923/j.chemistry.20120206.02.
Article Outline
1. Introduction
- Zinc acts an essential metal for number of enzymes involved in diverse biological processes but can be toxic at high concentrations[1]. Zinc has different enzymes that function in many aspects of cellular metabolism like proteins, lipids and carbohydrates metabolism[2], and anti-convulsant activities[3]. Many workers reported that the it has ability and flexibility to bind with amino acids to a variety of amino acid side-chains and substrates in a different geometry to enhance their biological activities[4-7]. Amino and carboxylic groups of amino acids that are implicated directly in enzymatic catalysis[8] with zinc metal as a function of active site of hydrolytic enzymes, where it is coordinated by N and O donor atoms[9]. Amino acids have been used as primary ligands and a large amount of research concerning metal amino acid complexation has been reported[10].Nicotinamide also binds amino acids and transition metal ions with very high affinity[11]. It is a pyridine derivative and important bioligand for human health and occurs in plants and human tissues[12]. The complexes formed with the amino acids and vitamin-PP (nicotinamide) with zinc (II) have been found great importance in many fields of biology, medicinal, organic and material chemistry, biochemistry cell biology[13,14] and many beneficial effects to providing better clinical recoveries and mobilization of heavy metal in vivo. In polarography, electrode kinetics is an area in which nature of electrode process has great importance. Tamamushi and Tanaka[15,16] determined the values of transfer coefficient(α), degree of irreversibility and rate constant(k) values. On the basis of α, we have located the exact position of transition state between oxidants and reductants [15,16]. The value of rate constant (k) measures the correct nature of electrode process at dropping mercury electrode.A systematic survey of literature reveals that no complex of zinc with selected L-amino acids and nicotinamide has been reported polarographically and hence the authors have undertaken the present study for their work.
2. Experimental
2.1. Materials
- All the solutions were prepared in doubled distilled water using AR grade chemicals. The metal used as Zinc Chloride from Aldrich, L-amino acids from Lobachem, vitamin-PP from Fluka. Potassium dihydrogen phosphate buffer was used to adjust the pH of the analyte. The concentrating of Zn (II), L-amino acids and vitamin-PP were taken in the ratio of 1:40:40 and current voltage curves were obtained at different pH values from 7 to 8.5, it has been observed that the maximum shift of E1/2 was observed at pH = 7.30 therefore; this pH was selected for the present study.
2.2. Instruments
- The current-voltage curves were recorded on a manual polarograph using polyflex galvanometer-PL-50. The polarographic cell was of Laitinen and Lingane type in which a polarographic capillary of 5.0 cm in length with 0.04 mm in diameter was used. The effective height of mercury m2/3 t1/6 value was 2.40 mg2/3s-1/2 at 60.02 cm. The Elico-pH Meter (Model LI-120) was used to measure the pH of the test solution at 7.30 ± 0.01.
2.3. Methods
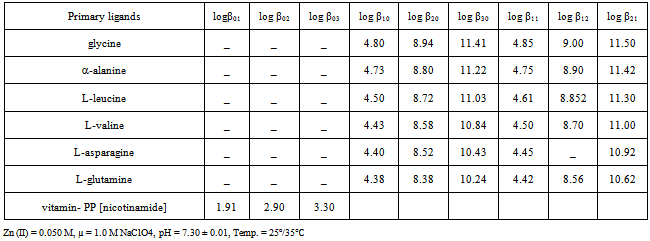
- The concentrations of zinc (II), NaClO4 and Triton X-100 (as suppressor) in the test solution were 0.50 mM, 1.0 M and 0.001 % respectively. For ternary system, the concentrations of primary ligands i.e. L-amino acids varied from 0.5 mM to 30.0 mM at 0.025 M and 0.050 M fixed concentration of secondary ligand vitamin- PP (nicotinamide) respectively. The E1/2 became more negative with the addition of secondary ligand (vitamin-PP) to the binary system [Zn-L-amino acids] showed ternary complex formation of 1: 1: 1, 1: 1: 2 and 1: 2: 1 complexes. The zinc (II) showed quasireversible reduction wave involving two electrons, at pH = 7.30 ± 0.01 and µ = 1.0 M NaClO4 at 25℃ and 35℃ [17,18]. The nature of current-voltage curves for complexes was also quasireversible. The stability constants of ternary systems are represented in table 1.
3. Results and Discussion
3.1. Polarographic Studies
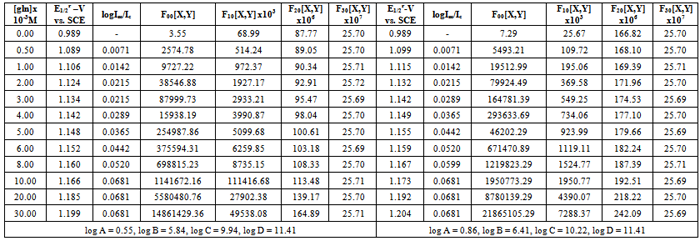
- The polarographic data and plots of Fij [X, Y] against [X] (where Fij is a Schaap and McMaster function to evaluate the stability constant βij, X = L-glycine, Y = vitamin-PP and i and j are their stoichiometric numbers respectively) for [Zn-L-glycine-vitamin-PP] system are shown in the table 2 and Figure 1 respectively [19]. The polarographic data are used to determine the values of function F00, F10, F20 and F30. The values of Er1/2 from Eqr1/2 were determined by Gellings [20] methods by plotting the graph between [E-RT/nF log (id-i)/i] vs. i for all the complexes were given in Figure 2 (a) and (b) respectively.
3.2. Comparison of the Stability of Binary Complexes and Ternary Complexes
- The relative stabilities of the binary and ternary complexes are quantitatively expressed in term log Km values which were calculated by the following equation[21]The values of logKm were 0.659, 649, 0.635, 0.617, 0.614 and 0.611 respectively for[Zn - L-glycinate - vitamin-PP], [Zn - α-alaninate - vitamin-PP], [Zn - L-leucinate - vitamin -PP], [Zn - L-valinate-vitamin-PP], [Zn - L-asparaginate – vitamin-PP] and [Zn - L-glutaminate - vitamin-PP] system respectively. The positive values of log km indicated that the ternary complexes are more stable than the corresponding binary complexes.
3.3. Trends of the Stability Constants of the Complexes
- The trend of stability constants of the complexes was found L-glutamine < L-asparagine < L-valine < L-leucine < α-alanine < L-glycine. The sequence may be explained[22] on the basis of basicities and structures of the primary ligands (L-amino acids). The metal ion zinc (II) is bound to the amino group and carboxyl group of the amino acids[23]. The stability constants of L-glycine complexes are maximum might be due to decreases in the side chain of L-amino acids than the rest of primary ligands. The stability of complexes decreases with the increases in the size of L-amino acids[24]. The values of stability constant (log β) varied from 1.91 to 11.50. The zinc (II) metal ion is coordinated through the nitrogen of pyridine group and oxygen of amide group in vitamin-PP to form a five membered ring[25].The values of stability constants of the complexes that vitamin-PP and amino acids used either singly or simultaneously might be effective to reduce the toxicity of metal in vivo.
|
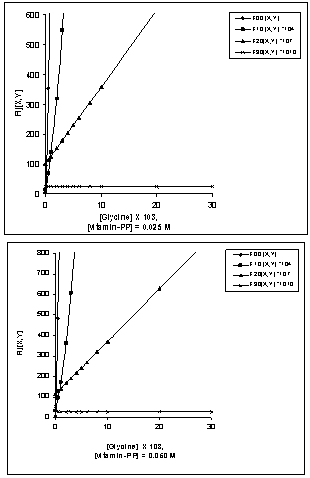 | Figure 1. Plots of Fij[X, Y] vs.[X] for[Zn -glycinate-vitamin-PP] system |
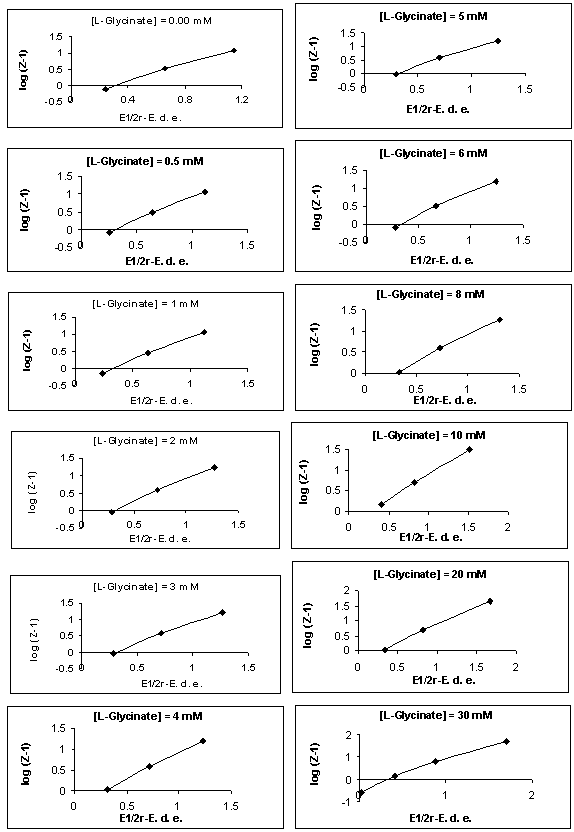 | Figure 2. (a). [Zn-glycinate-vitamin-PP],[vitamin-PP] = 0.025 M, Plots of (E1/2r -E) vs. log (Z-1), Y-axis = log (Z-1), X-axis = (E1/2r -E) |
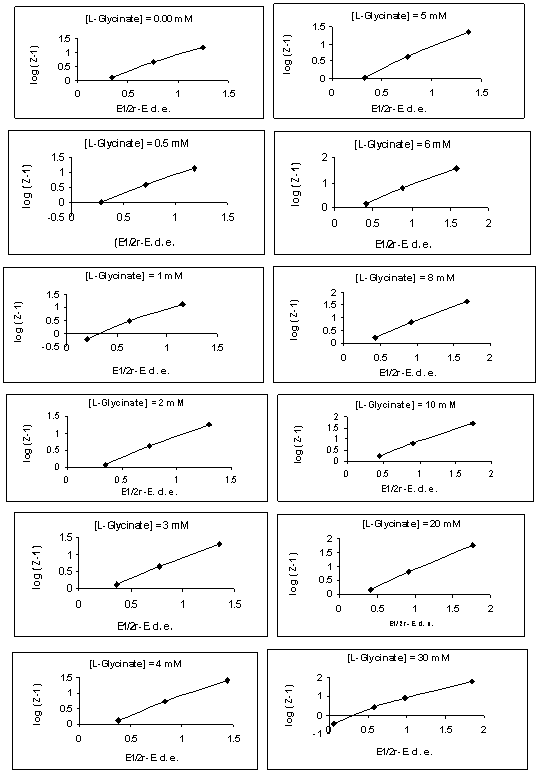 | Figure 2(b). [Zn-glycinate-vitamin-PP],[vitamin-PP] = 0.050 M, Plots of (E1/2r-E) vs. log (Z-1), Y-axis = log (Z-1), X-axis = (E1/2r -E) |
|
|
|
3.4. Kinetic Parameters and Thermodynamic Parameters
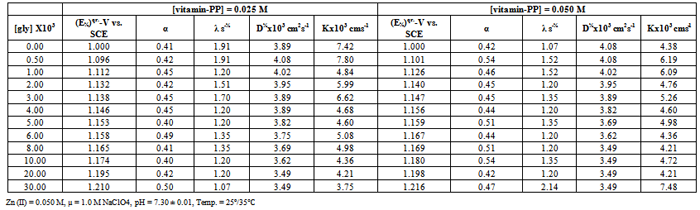
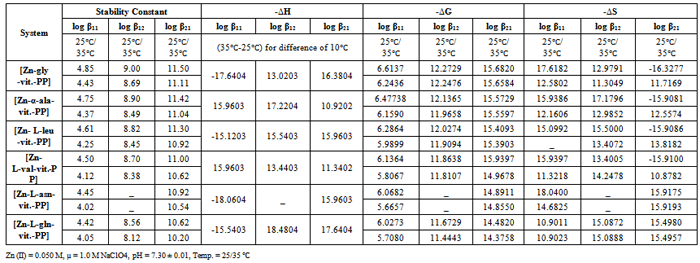
- The kinetic parameters of the complexes such as transfer coefficient (α), degree of irreversibility (λ) and rate constant (k) were determined by Tamamushi and Tanaka[15,16] methods and presented in the Table 3. The parameter Z, which measures the degree of irreversibility, has been calculated by the equation given in the literature[25]. The plots between (Er1/2-E) against log (Z-1) were given in Figure 3(a) and 3(b) respectively, where the terms have the usual significance.The thermodynamic parameters [19] such as enthalpy change (∆H), free energy change (∆G), and entropy change of the complexes are presented in the Table 4 and have been calculated by the equations given below
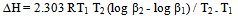 | (1) |
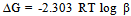 | (2) |
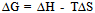 | (3) |
4. Conclusions
- Here we report the stability constants of [Zn – L-amino acidate – vitamin-PP] systems at pH = 7.30 ± 0.01, µ = 1.0 M NaClO4 at 25℃ and 35℃, polarographically. The thermodynamic and kinetic parameters were also reported. The result showed that the zinc complexes gave quasireversible reduction wave involving two electrons with diffusion controlled. The rate constant (k) determined the exact nature of electrode process on the basis of its values (k = 2x10-2- 5x10-5 cms-1) which is quasireversible. Also the transfer coefficient (α) determined about 0.50 which confirmed that the ‘transition state’lies in mid of the oxidant and reductant. Degree of irreversibility (λ) and diffusion coefficient (D) are also having the expected values. The knowledge of stability constants and kinetic parameters exploits in diverse fields in applied and theoretical chemistry.
ACKNOWLEDGEMENTS
- The authors are thankful to University Grant Commission, New Delhi for providing the Rajeev Gandhi fellowship to KR and the Head, Department of the Chemistry, Dr. H. S. Gour University, Sagar, for providing the laboratory facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML