-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(5): 277-281
doi: 10.5923/j.chemistry.20120205.06
Synthesis of Some New Functionalized Bis-thiazolidin-5-one and Bis-thiazolidin-4-one Derivatives
M. A. Metwally , A. Fekri , N. Nagy , F. A. Amer
Chemistry Department, Faculty of Science, MansouraUniversity, 35561, Mansoura, Egypt
Correspondence to: M. A. Metwally , Chemistry Department, Faculty of Science, MansouraUniversity, 35561, Mansoura, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A series of bis-thiazolidin-5-one and bis-thiazolidin-4-one derivatives 3and5 was prepared by the treatment of highly functional thiocarbamoyl intermediates 2 and 4 with chloroacetyl chloride and chloroacetic acid, respectively. Treatment of the bis-thiocarbamoyl derivative 4a with diazotized sulphanilic acid affected acetyl cleavage to afford the corresponding arylhydrazono-thiocarbamoyl derivative 6.The title compounds bis-thiazolidin-5-one and bis-thiazolidin-4-one derivatives showed high reactivity towards azo coupling reaction with diazotized sulphanilic acid and Knoevenagel reaction with 4-isopropylbenzaldehyde.
Keywords: Thiocarbamoyl,chloroacetyl chloride,bis-thiazolidin-5(and 4-)-ones,Japp-Klingmann reaction
Cite this paper: M. A. Metwally , A. Fekri , N. Nagy , F. A. Amer , "Synthesis of Some New Functionalized Bis-thiazolidin-5-one and Bis-thiazolidin-4-one Derivatives", American Journal of Chemistry, Vol. 2 No. 5, 2012, pp. 277-281. doi: 10.5923/j.chemistry.20120205.06.
1. Introduction
- The chemistry of thiazole derivatives, including new methodologies for their preparation, and recent applications, such as their growing use in organic synthesis in the biological field and asymmetric catalysis as ligands has been recently reviewed[1]. Thiazolidinones are known mainly as biologically active compounds with a broad range of activity and as intermediates in the synthesis of antibiotics and dyes[2-4]. Several papers have been published on the use of these compounds as antimicrobial[5,6], antifungal[7,8], anti-inflammatory activity[9], anticonvulsant[10],anesthetic[11] and antiviral drugs[12]. 2-Aminothiazoles and its derivatives are also used in the syntheses of various types of dyes[13-16]. In this work, a series of bis-thiazolidin-5-one and bis-thiazolidin-4-one derivatives was prepared by treatment of their corresponding thiocarbamoyl intermediates with chloroacetyl chloride and chloroacetic acid, respectively. Also, the reactions of title compounds bis-thiazolidin-one derivatives with diazotized sulphanilic acid and with 4-isopropylbezaldehyde were studied.
2. Materials and Methods
- All melting points were measured on an electrothermal Gallenkamp melting apparatus. Elemental analyses were carried out at the Microanalytical Unit, Faculty of Science, University of Mansoura, Egypt; the results were in satisfactory agreement with the calculated values. IR spectra (KBr) were determined on a Mattson 5000 FTIR spectrometer (not all frequencies are reported). Mass spectra were obtained at a Finnigan MAT 212 instrument (electron impact: 70 eV). The title compound 1 was prepared by boiling a hot solution of ethyl acetoacetate (7.6 ml, 0.06 mol) in 30 ml dry xylene with a hot solution of benzidine (5.5 g, 0.03 mol) in dry xylene (30 ml). The precipitate that formed on cooling was filtered off, dried and recrystallized from ethanol to give compound 1 as buff crystals, m.p. = 230 - 232℃, yield = 85%. IR (ν/cm-1): 3356 (NH), 1668 (C=O), 1633 (C=O). Elemental analysis: Calc. for C20H20O4N2 (352.38): C = 68.16, H = 5.72, N = 7.95%. Found: C = 68.29, H = 5.81, N = 7.88%.Synthesis ofN,N'-([1,1'-biphenyl]-4,4'-diyl)-bis-(3-oxo-2-(5-oxo-3-phenyl-thiazolidin-2-ylidene)butanamide) (3)To a cold suspension of finely divided KOH (0.78 g, 14 mmol) in DMF (20 ml) was added the title compound1 (2.46 g, 7 mmol) followed by phenyl isothiocyanate (1.68 ml, 14 mmol). The mixture was stirred at room temperature overnight, and then treated with chloroacetyl chloride (1.12 ml, 14 mmol). The stirring was continued at room temperature for 8 hours. The reaction mixture was poured into ice-cold water. The resultant solid product was collected by filtration, washed with water, dried, and recrystallized from ethanol. m.p. = 223 - 225℃, yield = 72%. IR (ν/cm-1): 3356 (NH), 1730 (C=O), 1660 (C=O), 1633 (C=O). MS: m/z = 702 (M+, 20), 400 (30), 298 (45), 226 (83), 103 (75). Elemental analysis: Calc. for C38H30N4O6S2 (702.8): C = 64.94, H = 4.30, N = 7.97%. Found: C = 65.08, H = 4.24, N = 7.89%.N,N'-([1,1'-biphenyl]-4,4'-diyl)-bis-(2-(aryl-thiocarbamoyl)-3-oxo-butanamide) derivatives (4)To a cold suspension of finely divided KOH (0.78 g, 14 mmol) in DMF (20 ml) were added the title compound1 (2.46 g, 7 mmol) followed by phenyl isothiocyanate (1.68 ml, 14 mmol). The reaction mixture was stirred at room temperature overnight, poured into ice-cold water, and then neutralized with dilute HCl. The resultant solid product was collected by filtration, washed with water, dried, and recrystallized from ethanol to afford compounds 4a and 4b. (4a): m.p. = 133 - 135°C, yield = 65%. IR (ν/cm-1): 3344(NH), 3202 (NH), 1665 (C=O), 1610 (C=N). Elemental analysis: Calc. for C34H30N4O4S2 (622.76): C = 65.57, H = 4.86, N = 9.00%. Found: C = 65.33, H = 4.77, N = 8.89%.(4b): m.p. = 119 - 120℃, yield = 78%. IR (ν/cm-1): 3367(NH), 3211 (NH), 1660 (C=O), 1607 (C=N). MS: m/z = 526 (M+, 15), 311 (25), 195 (40), 131 (90), 60 (100), 55 (53). Elemental analysis: Calc. for C26H30N4O4S2 (526.67): C = 59.29, H = 5.74, N = 10.64%. Found: C = 59.41, H = 5.88, N = 10.73%.Synthesis ofN,N'-([1,1'-biphenyl]-4,4'-diyl)-bis-(3-oxo-2-(4-oxo-thiazolidin-2-ylidene)-butanamide) derivatives (5)A mixture of bis-thiocarbamoyl4 (3 mmol) and choloroacetic acid (0.57 g, 6 mmol) was refluxed for 4-6 hours in glacial acetic acid (30 ml) containing fused sodium acetate (0.5 g, 6 mmol). The reaction mixture was cooled and poured into cooled water. The solid precipitate that formed was filtered off, dried and recrystallized from ethanol to afford the bis-thiazolidin-4-one derivatives 5. (5a): m.p. > 310℃, yield = 74%. IR (ν/ cm-1): 3410 (NH), 1715 (C=O), 1632 (C=O). MS: m/z = 702 (M+, 10), 386 (23), 236 (40), 158 (55), 73 (80), 50 (100). Elemental analysis: Calc. for C38H30N4O6S2 (702.8): C = 64.94, H = 4.30, N = 7.97%. Found: C = 64.83, H = 4.36, N = 7.88%.(5b): m.p. > 312°C, yield = 70%. IR (ν/cm-1): 3434 (NH), 1714 (C=O), 1631 (C=O).MS: m/z = 606 (M+, 10), 213 (14), 133 (25), 106 (52), 57 (100).Elemental analysis: Calc. for C30H30N4O6S2 (606.71): C = 59.39, H = 4.98, N = 9.23%. Found: C = 59.58, H = 5.05, N = 9.32%.Reaction of bis-thiocarbamoyl derivative 4a with diazotized sulphanilic acid Formation of (6)A solution of compound 4a (2.48 g, 4 mmol) in ethanol (20ml) and sodium acetate (1.5 g) was stirred in an ice – bath at 0-5℃. Diazotized solution of sulphanilic acid (1.38 g, 8 mmol) was added to stirred solution over a period of 30 minutes and the stirring was continued for 2 hours with cooling. The reaction mixture was left in ice – bath and the formed solid product was filtered off, dried and recrystallized from ethanol to afford compound 6.m.p. > 350℃, yield = 71%. IR (ν/cm-1): 3326 (NH), 3216 (NH), 1631 (C=O). MS: m/z = 907 (M+, 10), 382 (20), 193 (40), 113 (44), 58 (57), 55 (100). Elemental analysis: Calc. for C42H34N8O8S4 (907.03): C = 55.62, H = 3.78, N = 12.35%. Found: C = 55.40, H = 3.85, N = 12.41%.Formation of compounds 7 and 8The diazotized solution of sulphanilic acid (0.69 g, 2 mmol) was added with continuous stirring to a cold solution of compound 3 or compound 5a (4 mmol) in 20 ml ethanol containing sodium acetate (0.75 g). The reaction mixture was stirred at 0-5℃ for 2 hours, left to stand at room temperature. The solid product that obtained was filtered off, dried and recrystallized from ethanol to afford compound 7 or compound 8, respectively.(7): m.p. = 235 – 237℃, yield = 73%. IR (ν/cm-1): 3430 (NH), 1688 (C=O), 1657 (C=O). MS: m/z = 1071 (M+, 15), 974 (18), 408 (25), 271 (33), 120 (63), 58 (100), 53 (66). Elemental analysis: Calc. for C50H38N8O12S4 (1071.14): C = 56.06, H = 3.58, N = 10.46%. Found: C = 56.32, H = 3.62, N = 10.24%.(8): m.p. > 320℃, yield = 76%. IR (ν/cm-1): 3395 (NH), 1673 (C=O), 1633 (C=O). MS: m/z = 1071 (M+, 5), 980 (23), 311 (33), 135 (55), 79 (100), 65 (73). Elemental analysis: Calc. for C50H38N8O12S4 (1071.14): C = 56.06, H = 3.58, N = 10.46%. Found: C = 56.18, H = 3.46, N = 10.37%.Formation of compounds 9 and 10A mixture of bis-thiazolidin-5-one derivative 3 or bis-thiazolidin-4-one derivative 5a(3 mmol) and 4-isopropylbenzaldehyde (0.9 ml, 6 mmol) was refluxed for 4 hours in ethanol (30 ml) containing catalytic amount of piperidine. The solid products that formed on cooling were filtered off, dried and recrystallized from ethanol to afford the corresponding arylidene derivatives 9 or 10, respectively. (9): m,p. = 310 – 312℃, yield = 78%. IR (ν/cm-1): 3362 (NH), 1678 (C=O), 1654 (C=O). MS: m/z = 963 (M+, 30), 893 (45), 507 (35), 210 (40), 184 (100). Elemental analysis: Calc. for C58H50N4O6S2 (963.17): C = 72.33, H = 5.23, N = 5.82%. Found: C = 72.44, H = 5.27, N = 5.70%. (10): m.p. > 350℃, yield = 73%. IR (ν/cm-1): 3380 (NH), 1666 (C=O), 1643 (C=O). MS: m/z = 963 (M+, 15), 601 (20), 386 (100), 236 (77), 92 (95), 51 (82). Elemental analysis: Calc. for C58H50N4O6S2 (963.17): C = 72.33, H = 5.23, N = 5.82%. Found: C = 72.24, H = 5.18, N = 5.88%.
3. Results and Discussions
- The highly versatile compoundN,N'-([1,1'-biphenyl]-4,4'-diyl)bis(3-oxobutanamide) (1) was prepared from benzidine and ethyl acetoacetate . The base-prompted reaction of the acidic methylene compound 1 with phenyl isothiocyanate in dry DMF at room temperature in basic medium led to the formation of the non-isolable intermediates 2 which underwent insituheterocyclization reaction with chloroacetyl chloride to afford the corresponding bis-thiazolidin-5-one derivative 3 (Scheme 1). Insitu treatment of the non-isolable intermediates 2 with dilute HCl gave the corresponding bis-thiocarbamoyl derivatives 4a and 4b. Refluxing of 4 with chloroacetic acid in acetic acid containing anhydrous sodium acetate afforded the corresponding bis-thiazolidin-4-one derivatives 5. The molecular structure of 5 was confirmed by analytical and spectral data. Treatment of bis-thiocarbamoyl derivative 4a with diazotized sulphanilic acid in the presence of sodium acetate affected acetyl cleavage (Japp-Klingmann reaction type) with the formation of arylhydrazono-thiocarbamoyl derivative 6 (Scheme 2). The structure of the highly functionalizedarylhydrazono-thiocarbamoyl derivative 6 was elucidated on the basis of its elemental analysis and spectral data.The reactivity of the methylene group in bis-thiazolidin-5-one derivative 3 and bis-thiazolidin-4-one derivative 5a was tested toward the azo coupling reactionwith diazonium salts. Thus, when two moles of diazotized sulphanilic acid at 0-5℃ reacted with the thiazolidinonederivatives 3 and 5a yielded the corresponding bis-hydrazono derivatives 7 and 8 respectively. The structures of compounds 7 and 8 were assigned on the basis of their elemental analyses and spectral data.Treatment of bis-thiazolidin-5-one derivative 3 and bis-thiazolidin-4-one derivative 5a withp-isopropylbenzaldehyde in ethanol/piperidine afforded the corresponding bis-(4-arylidene-thiazolidin-5-one) 9 andbis-(5-arylidene-thiazolidin-4-one) 10 in good yields. Assignment of the products 9 and 10 was based on their elemental analyses and spectral data.
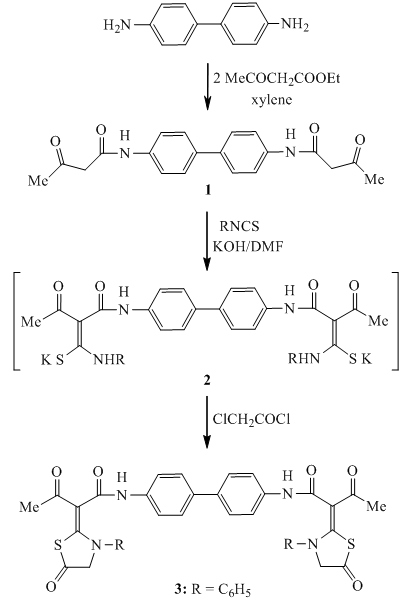 | Scheme 1. Synthesis ofbis-thiazolidin-4-one derivative 3 |
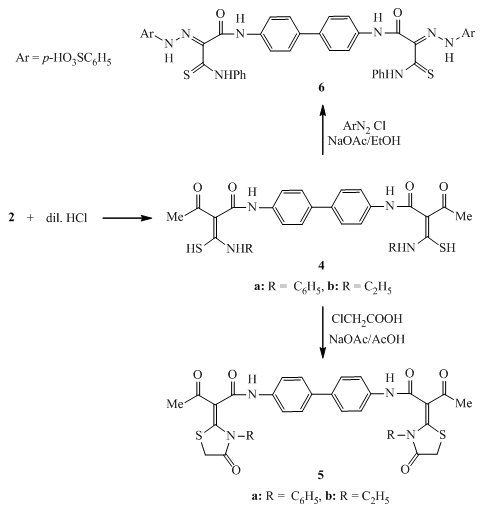 | Scheme 2. Synthesis ofbis-thiazolidin-4-one derivatives 5 and arylhydrazono-thiocarbamoyl derivative 6 |
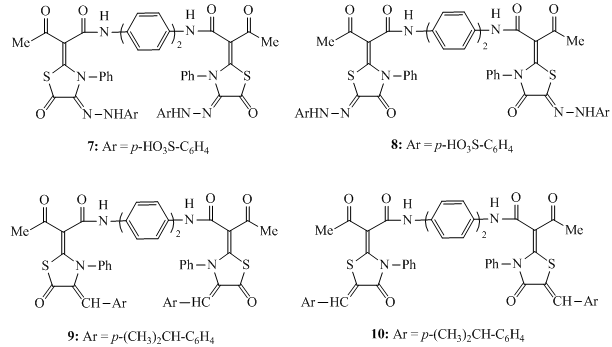 | Scheme 3. Reactions of thiazolidin-5-one derivatives 3 and 5a with diazotized sulphanilic acid and p-isopropylbenzaldehyde |
4. Conclusions
- We have synthesized new bis-thiazolidin-5-one and bis-thiazolidin-4-one derivatives by the reaction of available thiocarbamoyl derivatives with chloroacetyl chloride and chloroacetic acid, respectively.The title compounds bis-thiazolidin-5-one and bis-thiazolidin-4-one derivatives showed high reactivity towards azo coupling reaction with diazotized sulphanilic acid and Knoevenagel reaction with 4-isopropylbenzaldehyde.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML