-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(5): 263-270
doi: 10.5923/j.chemistry.20120205.04
Comparative Study of Duolite A-378 and Duolite A-143 Anion Exchange Resins by Application of 131I and 82 Br as a Tracer Isotopes
Pravin U. Singare
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, 400 058, India
Correspondence to: Pravin U. Singare , Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, 400 058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the present study radioactive tracer isotopes 131I and 82Br are applied to study the kinetics of iodide and bromide ion-isotopic exchange reactions in two anion exchange resins Duolite A-378 and Duolite A-143. The study is performed by varying the experimental parameters like temperature and concentration of exchanging medium to understand the effect of above parameters on exchange reaction rate (min-1), amount of ions exchanged (mmol), percentage of ions exchanged and distribution coefficient. For both the ion-isotopic exchange reactions it is common observation that the exchange reaction rate increases with increase in concentration and decreases with rise in temperature of exchanging medium. Also there exists a strong positive co-relationship between amount of ions exchanged and concentration of ionic solution, while a strong negative co-relationship exists between amounts of ions exchanged and temperature of exchanging medium. Comparing the values of reaction rate (min-1), amount of ions exchanged (mmol); percentage of ions exchanged and distribution coefficient calculated for the two resins, it is observed that Duolite A-378 resins show superior performance than Duolite A-143 resins under identical operational parameters.
Keywords: Duolite A-378, Duolite A-143, Reaction Kinetics, Radioactive Isotopes, Tracer Applications, Distribution Coefficient, Ion-Isotopic Exchange Reactions, Reaction Rate
Cite this paper: Pravin U. Singare , "Comparative Study of Duolite A-378 and Duolite A-143 Anion Exchange Resins by Application of 131I and 82 Br as a Tracer Isotopes", American Journal of Chemistry, Vol. 2 No. 5, 2012, pp. 263-270. doi: 10.5923/j.chemistry.20120205.04.
Article Outline
1. Introduction
- Radio isotopes hold a central place in atomic energy programme as the major non-power application of nuclear industry [1]. Radio isotope as a tracers offer several advantages in radiochemical investigation such as high detection sensitivity, capability of in-situ detection, limited memory effects and physico-chemical compatibility with the material under study. The fundamental principle in radiochemical investigations is that the chemical properties of a radio isotope of an element are almost the same as those of the other stable/radioactive isotopes of the element. When radio isotope is present in a chemical form identical to that of the bulk of the element in a chemical process, then any reaction the element undergoes can be directly traced by monitoring the radio isotope. Radiochemical work involves two main steps first is the sampling of chemical species to be studied and second is quantitative determination of the radiation emitted by the radio isotope in the sample [1]. In radiotracer study, a short lived radio isotope in a physico-chemical form similar to that of the process material is used to trace the material under study. The radio isotopes in suitable physical and chemical forms are introduced in systems under study. By monitoring the radioactivity both continuously or after sampling (depending on the nature of study), the movement, adsorption, retention etc. of the tracer and in turn, of the bulk matter under investigation, can be followed. The tracer concentration recorded at various locations also helps to draw information about the dynamic behaviour of the system under study. The radio isotopes preferred for such studies are gamma emitters having half-life compatible with the duration of studies. The strength of radioactivity used varies depending on the nature of application. Radiotracer methodology is described extensively in the literature [2-6]. Applications of radiotracers in chemical research cover the studies of reaction mechanism, kinetics, exchange processes and analytical applications such as radiometric titrations, solubility product estimation, isotope dilution analysis and autoradiography. The radio isotopes have proved as a tool to study many problems in chemical, biological and medicinal fields. Radiotracers have helped in identification of leaks in buried pipelines and dams. Process parameters such as mixing efficiency, residence time, flow rate, material inventory and silt movement in harbours are studied using radio isotopes[1].The efficiency of several devices in a wastewater treatment plant (primary and secondary clarifiers, aeration tank) is investigated by means of radiotracers[7]. Radio isotopes play an important role in a variety of fields such as health-care, agriculture, industry, hydrology, life sciences, pollution control and research. The radio isotopes have also proved a tool to study many problems in chemical, biological and medicinal fields. Considering the above wide use of radioactive isotopes in various industrial and technical applications, in the present investigation, they are applied to assess the performance of industrial grade anion exchange resins Duolite A-378 and Duolite A-143 under different operational parameters like temperature and ionic concentrations. It is expected that the tracer technique used here can also be used for characterization of other organic ion exchange resins which are synthesized for their specific technical applications [8-10]. The present technique can also be extended further to standardize the operational parameters so as to bring about the most efficient performance of those resins in their specific industrial applications.
2. Experimental
2.1. Conditioning of Ion Exchange Resins
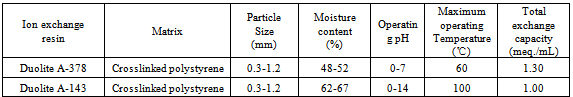
- Ion exchange resin Duolite A-378 is a weak base anion exchange resin in OH- form having-N+R2 functional group, while Duolite A-143 is a strongly basic anion exchange resin in Cl- form having -N+R3 functional group (supplied by Auchtel Product Ltd., Mumbai, India). Details regarding the properties of the resins used are given in Table 1. These resins were converted separately in to iodide / bromide form by treatment with 10 % KI / KBr solution in a conditioning column which is adjusted at the flow rate as 1 mL / min. The resins were then washed with double distilled water, until the washings were free from iodide/bromide ions as tested by AgNO3 solution. These resins in bromide and iodide form were then dried separately over P2O5 in desiccators at room temperature.
2.2. Radioactive Tracer Isotopes
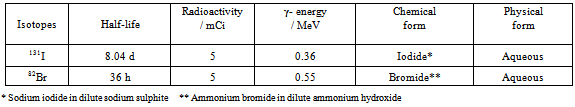
- The radioisotope 131I and 82Br used in the present experimental work was obtained from Board of Radiation and Isotope Technology (BRIT), Mumbai. Details regarding the isotopes used in the present experimental work are given in Table 2.
2.3. Study on kinetics of Iodide Ion-Isotopic Exchange Reaction
- In a stoppered bottle 250 mL (V) of 0.001 M iodide ion solution was labeled with diluted 131I radioactive solution using a micro syringe, such that 1.0 mL of labeled solution has a radioactivity of around 15,000 cpm (counts per minute) when measured with γ -ray spectrometer having NaI (Tl) scintillation detector. Since only about 50–100 μL of the radioactive iodide ion solution was required for labelling the solution, its concentration will remain unchanged, which was further confirmed by potentiometer titration against AgNO3 solution. The above labeled solution of known initial activity (Ai) was kept in a thermostat adjusted to 30.0 ℃. The swelled and conditioned dry ion exchange resins in iodide form weighing exactly 1.000 g (m) were transferred quickly into this labeled solution which was vigorously stirred by using mechanical stirrer and the activity in cpm of 1.0 mL of solution was measured. The solution was transferred back to the same bottle containing labeled solution after measuring activity. The iodide ion-isotopic exchange reaction can be represented as:
 | (1) |
|
|
|
2.4. Study on Kinetics of Bromide Ion-Isotopic Exchange Reaction
- The experiment was also performed to study the kinetics of bromide ion- isotopic exchange reaction by equilibrating 1.000 g of ion exchange resin in bromide form with labeled bromide ion solution in the same concentration and temperature range as above. The labelling of bromide ion solution was done by using 82Br as a radioactive tracer isotope for which the same procedure as explained above was followed. The bromide ion-isotopic exchange reaction can be represented as:
 | (2) |
3. Results and Discussion
3.1. Comparative Study of Ion-Isotopic Exchange Reactions
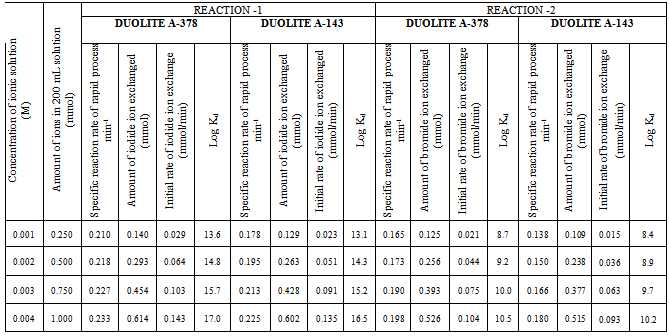
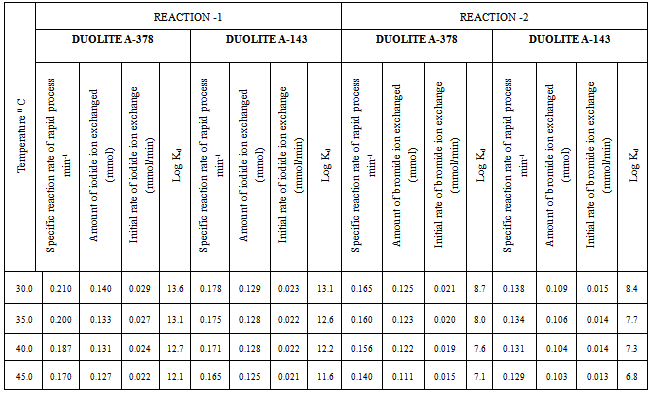
- In the present investigation it was observed that due to the rapid ion-isotopic exchange reaction taking place, the activity of solution decreases rapidly initially, then due to the slow exchange the activity of the solution decreases slowly and finally remains nearly constant. Preliminary studies show that the above exchange reactions are of first order [27, 28]. Therefore logarithm of activity when plotted against time gives a composite curve in which the activity initially decreases sharply and thereafter very slowly giving nearly straight line (Figure 1), evidently rapid and slow ion-isotopic exchange reactions were occurring simultaneously[12-26]. Now the straight line was extrapolated back to zero time. The extrapolated portion represents the contribution of slow process to the total activity which now includes rapid process also. The activity due to slow process was subtracted from the total activity at various time intervals. The difference gives the activity due to rapid process only. From the activity exchanged due to rapid process at various time intervals, the specific reaction rates (k) of rapid ion-isotopic exchange reaction were calculated. The amount of iodide / bromide ions exchanged (mmol) on the resin were obtained from the initial and final activity of solution and the amount of exchangeable ions in 250 mL of solution. From the amount of ions exchanged on the resin (mmol) and the specific reaction rates (min-1), the initial rate of ion exchanged (mmol/min) was calculated. Because of larger solvated size of bromide ions as compared to that of iodide ions, it was observed that the exchange of bromide ions occurs at the slower rate than that of iodide ions [29]. Hence under identical experimental conditions, the values of specific reaction rate (min-1), amount of ion exchanged (mmol) and initial rate of ion exchange (mmol/min) are calculated to be lower for bromide ion-isotopic exchange reaction than that for iodide ion-isotopic exchange reaction as summarized in Tables 3 and 4. For both bromide and iodide ion-isotopic exchange reactions, under identical experimental conditions, the values of specific reaction rate increases with increase in concentration of ionic solution from 0.001M to 0.004M (Table 3). However, with rise in temperature from 30.0℃ to45.0℃, the specific reaction rate was observed to decrease (Table 4). From the results, it appears that iodide ions exchange at the faster rate as compared to that of bromide ions which was related to the extent of solvation (Tables 3 and 4).
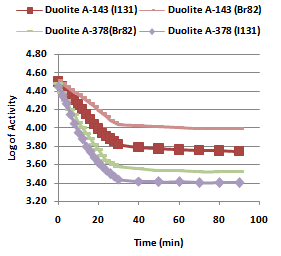 | Figure 1. Kinetics of Ion-Isotopic Exchange Reactions Amount of ion exchange resin = 1.000 g, Concentration of labeled exchangeable ionic solution = 0.001M, Volume of labeled ionic solution = 250 mL, Temperature = 30.0℃ |
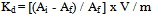 | (3) |
3.2. Comparative study of Anion Exchange Resins
- From the Table 3 and 4, it is observed that for iodide ion-isotopic exchange reaction by using Duolite A-378 resin, the values of specific reaction rate (min-1), amount of iodide ion exchanged (mmol), initial rate of iodide ion exchange (mmol/min) and log Kd were 0.210, 0.140, 0.029 and 13.6 respectively, which was higher than 0.178, 0.129, 0.023 and 13.1 respectively as that obtained by using Duolite A-143 resins under identical experimental conditions of 30.0℃, 1.000 g of ion exchange resins and 0.001 M labeled iodide ion solution. The identical trend was observed for the two resins during bromide ion-isotopic exchange reaction. From Table 3, it is observed that using Duolite A-378 resins, at a constant temperature of 30.0℃, as the concentration of labeled iodide ion solution increases 0.001 M to 0.004 M, the percentage of iodide ions exchanged increases from 56.1 % to 61.4 %. While using Duolite A-143 resins under identical experimental conditions the percentage of iodide ions exchanged increases from 51.4 % to 60.2 %. Similarly in case of bromide ion-isotopic exchange reaction, the percentage of bromide ions exchanged increases from 50.2 % to 52.6 % using Duolite A-378 resin, while for Duolite A-143 resin it increases from 43.7 % to 51.5 %. The effect of ionic concentration on percentage of ions exchanged is graphically represented in Figure 2.
 | Figure 2. Variation in Percentage Ions Exchanged with Concentration of Labeled Ionic Solution Amount of ion exchange resin = 1.000 g, Volume of labeled ionic solution = 250 mL, Temperature = 30.0℃ |
 | Figure 3. Variation in Percentage Ions Exchanged with Temperature of labeled Ionic Solution Amount of ion exchange resin = 1.000 g, Concentration of labeled exchangeable ionic solution = 0.001M, Volume of labeled ionic solution = 250 mL, Amount of exchangeable ions in 250 mL labeled solution = 0.250 mmol |
3.3. Statistical Correlations
- The results of present investigation show a strong positive linear co-relationship between amount of ions exchanged and concentration of ionic solution (Figures 4, 5). In case of iodide ion-isotopic exchange reaction, the values of correlation coefficient (r) were calculated as 0.9999 and 0.9983 for Duolite A-378 and Duolite A-143 resins respectively, while for bromide ion-isotopic exchange reaction, the respective values of r was calculated as 1.0000 and 0.9999.
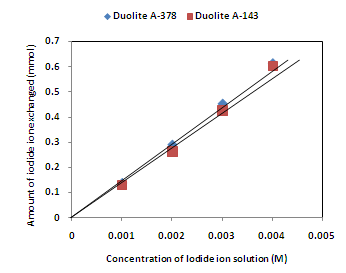 | Figure 4. Correlation between concentrations of iodide ion solution and amount of iodide ion exchanged Amount of ion exchange resin = 1.000 g, Volume of labeled ionic solution = 250 mL, Temperature = 30.0 0C |
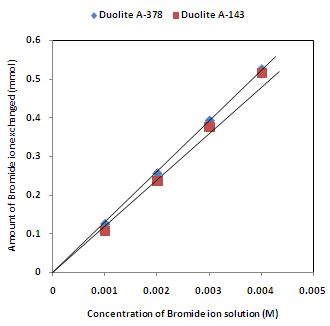 | Figure 5. Correlation between concentrations of bromide ion solution and amount of bromide ion exchanged Amount of ion exchange resin = 1.000 g, Volume of labeled ionic solution = 250 mL, Temperature = 30.0℃ |
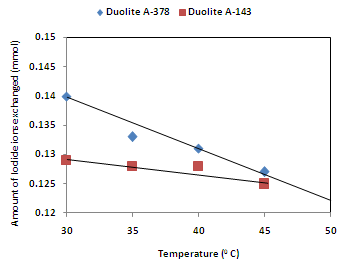 | Figure 6. Correlation between Temperatures of exchanging medium and amount of iodide ion exchanged Amount of ion exchange resin = 1.000 g, Concentration of labeled exchangeable ionic solution = 0.001M, Volume of labeled ionic solution = 250 mL, Amount of exchangeable ions in 250 mL labeled solution = 0.250 mmol |
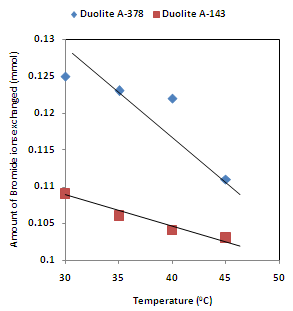 | Figure 7. Correlation between Temperatures of exchanging medium and amount of bromide ion exchanged Amount of ion exchange resin = 1.000 g, Concentration of labeled exchangeable ionic solution = 0.001M, Volume of labeled ionic solution = 250 mL, Amount of exchangeable ions in 250 mL labeled solution = 0.250 mmol |
4. Conclusions
- The experimental work carried out in the present investigation will help to standardize the operational process parameters so as to improve the performance of selected ion exchange resins. The radioactive tracer technique used here can also be applied for characterization of different nuclear as well as non-nuclear grade ion exchange resins.
ACKNOWLEDGEMENTS
- The author is thankful to Professor Dr. R.S. Lokhande for his valuable help and support in carrying out the experimental work in Radiochemistry Laboratory of Department of Chemistry, University of Mumbai, Vidyanagari, Mumbai -58.The author is extremely thankful to SAP Productions for developing and maintaining the manuscript template.
References
| [1] | Hou, X., Celler, A., Grimes, J., Benard, F., and Ruth, T., 2012, Theoretical dosimetry estimations for radioisotopes produced by proton-induced reactions on natural and enriched molybdenum targets., Physics in Medicine and Biology, 57(6), 1499-1515. |
| [2] | Sadeghi, M., Enferadi, M., and Nadi, H., 2010, Study of the cyclotron production of 172Lu: An excellent radiotracer., J. Radioanalytical and Nuclear Chemistry, 286(1), 259-263. |
| [3] | Kong, F.L., Ali, M.S., Rollo, A., Smith, D.L., Zang, Y., Yu, D.F., and Yang, D.J., 2012, Development of tyrosine-based radiotracer 99mTc-N4-tyrosine for breast cancer imaging., J. Biomedicine and Biotechnology, 2012, Article number 671708. |
| [4] | Koron, N., Bratkic, A., Ribeiro Guevara, S., Vahcic, M., and Horvat, M., 2012, Mercury methylation and reduction potentials in marine water: An improved methodology using 197Hg radiotracer., Applied Radiation and Isotopes, 70(1), 46-50. |
| [5] | Dagadu, C.P.K., Akaho, E.H.K., Danso, K.A., Stegowski, Z., and Furman, L., 2012, Radiotracer investigation in gold leaching tanks., Applied Radiation and Isotopes, 70(1), 156-161. |
| [6] | Makkar, H.P.S., 2008, A review of the use of isotopic and nuclear techniques in animal production., Animal Feed Science and Technology, 140(3-4), 418-443. |
| [7] | Milton, B.F., and Stevenson, N.R., 1995, Cyclotrons for isotope production., Proceedings of the IEEE Particle Accelerator Conference, Dallas, TX, USA, 1, 89-91. |
| [8] | Bahadur Aruna, 1985, Radio Isotopes as a Tool in Metallurgical Research and Industry., Transactions of the Indian Institute of Metals, 38(4), 301-308. |
| [9] | Reisener, H., 1969, General Introduction into Industrial Radiography., Founding Welding Production Eng J., 9(10), 27-45. |
| [10] | Sood, D.D., Reddy, A.V.R., and Ramamurthy, N., 2004, Applications of Radioisotopes In Physico-Chemical Investigations, in Fundamentals of Radiochemistry, Indian Association of Nuclear Chemists and Allied Scientists (IANCAS), 253-263. |
| [11] | Radiotracer Applications in Industry- A Guidebook, 2004, Safety Reports Series No. 423, International Atomic Energy Agency (IAEA) Vienna. |
| [12] | Murakami, K. Yu, X., Kato, T., Inoue, Y., and Sugawara, K., 2012, Synthesis of temperature- responsive anion exchanger via click reaction., Journal of Colloid and Interface Science, 376(1), 189-195. |
| [13] | Zhang, F., Li, Y., Guo, Z., Liang, T., Yang, B., Zhou, Y., and Liang, X., 2011, A polar-copolymerized method to prepare silica-based anion exchanger for ion chromatography., Talanta,85(1), 112-116. |
| [14] | Chen, S., Yue, Q., Gao, B., Li, Q., and Xu, X., 2011, Preparation and characteristics of anion exchanger from corn stalks., Desalination, 274(1-3), 113-119. |
| [15] | Sood, D.D., 1998, Proc. Int. Conf. on Applications of Radioisotopes and Radiation in Industrial Development, ed. Sood, D.D., Reddy, A.V.R., Iyer, S.R.K., Gangadharan, S., and Singh, G., (B.A.R.C., India) 35–53. |
| [16] | Singare, P.U., and Lokhande, R.S., 2012, Studies on Ion-Isotopic Exchange Reactions Using Nuclear Grade Ion Exchange Resins., Ionics, 18(4), 351–357. |
| [17] | Lokhande, R.S., Singare, P.U., and Kolte, A.R., 2010, Application of Radioactive Tracer Technique for Characterization of Strongly Basic Anion Exchange Resins Duolite A 101D and Duolite A 102D., Radiochemistry, 52(1), 81–86. |
| [18] | Lokhande, R.S., Singare, P.U., Tiwari, S.R.D., 2009, Application of 82Br as a Radioactive Tracer Isotope to Study the Bromide Ion-Isotopic Exchange Reaction in Strongly Basic Anion Exchange Resin Duolite – A161., Russ. J.Physical Chemistry, 83(8), 1389-1394. |
| [19] | Lokhande, R.S, Singare, P.U., and Tiwari, S.R.D., 2008, Study of Bromide Ion- Isotopic Exchange Reaction Kinetics Using a weakly Basic Macro porous Resin Indion – 860., Radiochemistry, 50(6), 633-637. |
| [20] | Lokhande, R.S., Singare, P.U., and Parab, S.A., 2008, Application of Radioactive Tracer Technique to Study the Kinetics of Iodide Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Duolite A-116., Radiochemistry, 50(6), 642-644. |
| [21] | Lokhande, R.S., Singare, P.U., and Patil, V.V., 2008, Application of Radioactive Tracer Technique to Study the Kinetics and Mechanism of Reversible Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Indion -850., Radiochemistry,50(6), 638-641. |
| [22] | Lokhande, R.S., Singare, P.U., and Prabhavalkar, T.S., 2008, The Application of the Radioactive Tracer Technique to Study the Kinetics of Bromide Isotope Exchange Reaction with the Participation of Strongly Basic Anion Exchange Resin Indion FF-IP., Russ. J. Physical Chemistry A, 82(9), 1589–1595. |
| [23] | [Singare, P.U., Lokhande, R.S., and Patil, A.B., 2008, Application of Radioactive Tracer Technique for Characterization of some Strongly Basic Anion Exchange Resins., Radiochim. Acta, 96(2), 99-104. |
| [24] | Lokhande, R.S., and Singare, P.U., 2008, Comparative Study on Iodide and Bromide Ion-Isotopic Exchange Reactions by Application of Radioactive Tracer Technique., J.Porous Mater., 15(3), 253-258. |
| [25] | Lokhande, R.S., Singare, P.U., and Patil, A.B., 2007, Application of Radioactive Tracer Technique on Industrial Grade Ion Exchange Resins Indion-830 (Type-1) and Indion-N-IP (Type-2)., Radiochim. Acta, 95(1), 111-114. |
| [26] | Lokhande, R.S., and Singare, P.U., 2007, Comparative Study on Ion-Isotopic Exchange Reaction Kinetics by Application of Tracer Technique., Radiochim. Acta, 95(3), 173-176. |
| [27] | Lokhande, R.S., Singare, P.U., and Kolte, A.R., 2007, Study on Kinetics and Mechanism of Ion-Isotopic Exchange Reaction Using Strongly Basic Anion Exchange Resins Duolite A-101 D and Duolite A-102 D., Radiochim. Acta, 95(10), 595-600. |
| [28] | Lokhande, R.S., Singare, P.U., and Dole, M.H., 2007, Application of Radiotracer Technique to Study the Ion Isotope Exchange Reactions Using a Strongly Basic Anion-Exchange Resin Duolite A-113., Radiochemistry, 49(5), 519-522. |
| [29] | Lokhande, R.S., Singare, P.U., and Karthikeyan, P., 2007, The Kinetics and Mechanism of Bromide Ion Isotope Exchange Reaction in Strongly Basic Anion-Exchange Resin Duolite A-162 Determined by the Radioactive Tracer Technique., Russ. J. Physical Chemistry A, 81(11), 1768–1773. |
| [30] | Lokhande, R.S., Singare, P.U., and Dole, M.H., 2006, Comparative Study on Bromide and Iodide Ion-Isotopic Exchange Reactions Using Strongly Basic Anion Exchange Resin Duolite A-113., J. Nuclear and Radiochemical Sciences, 7(2), 29-32. |
| [31] | Lokhande, R.S., and Singare, P.U., 2003, Study of reversible ion-isotopic self diffusion reaction using 82 Br as a radioactive tracer isotope., Asian J. Chem., 15(1), 33-37. |
| [32] | Lokhande, R.S., and Singare, P.U., 2005, Study on kinetics of self diffusion reaction by application of 82 Br as a radioactive tracer isotope., Asian J. Chem., 17(1), 125-128. |
| [33] | Shannon, R.D., 1976, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides., Acta Crystallographica, A32, 751-767. |
| [34] | Heumann, K.G., and Baier, K., 1982, Chloride distribution coefficient on strongly basic anion- exchange resin: Dependence on co-ion in alkali fluoride solutions., Chromatographia, 15(11), 701-703. |
| [35] | Adachi, S., Mizuno, T., and Matsuno, R., 1995, Concentration dependence of the distribution coefficient of maltooligosaccharides on a cation-exchange resin., J. Chromatography A, 708(2), 177-183. |
| [36] | Shuji, A., Takcshi, M., and Ryuichi, M., 1996, Temperature dependence of the distribution coefficient of maltooligosaccharides on cation-exchange resin in Na+ form., Bioscience, Biotechnology, and Biochemistry, 60(2), 338-340. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML