-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(5): 248-254
doi:10.5923/j.chemistry.20120205.02
An Easy and Efficient Protocol for the Synthesis of 2,3-Dihydroquinazolinones Using a Low Cost and Reusable Heterogeneous Catalyst
Bashir A. Dar1, Akshya K. Sahu1, Praveen Patidar1, jyoti patial1, Parveen Sharma1, Meena Sharma2, Baldev Singh1
1NPC & Catalysis, Indian Institute of Integrative Medicine (CSIR) ,Canal Road Jammu 180001,(J & K) India
2Department of Chemistry University of Jammu. (J & K) 180004, India
Correspondence to: Bashir A. Dar, NPC & Catalysis, Indian Institute of Integrative Medicine (CSIR) ,Canal Road Jammu 180001,(J & K) India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
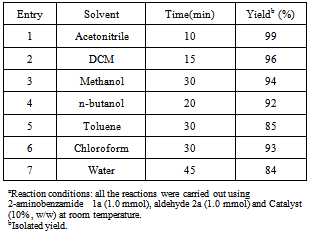
Clays revealed high catalytic activity for the synthesis of a series of substituted 2, 3-dihydro-2-phenylquinazolin-4(1H)-one. The synthesis was carried out by reacting Anthranilamide and various substituted aldehydes in acetonitrile at room temperature in the presence of a small amount of the catalyst. Several solvents were examined for this reaction; however, in terms of reaction yield and time, Acetonitrile was found to be the optimum solvent.
Keywords: Condensation, Cyclization, Heterogeneous Catalysis, Clay
Cite this paper: Bashir A. Dar, Akshya K. Sahu, Praveen Patidar, jyoti patial, Parveen Sharma, Meena Sharma, Baldev Singh, An Easy and Efficient Protocol for the Synthesis of 2,3-Dihydroquinazolinones Using a Low Cost and Reusable Heterogeneous Catalyst, American Journal of Chemistry, Vol. 2 No. 5, 2012, pp. 248-254. doi: 10.5923/j.chemistry.20120205.02.
Article Outline
1. Introduction
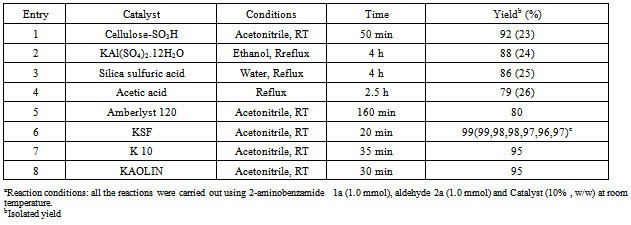
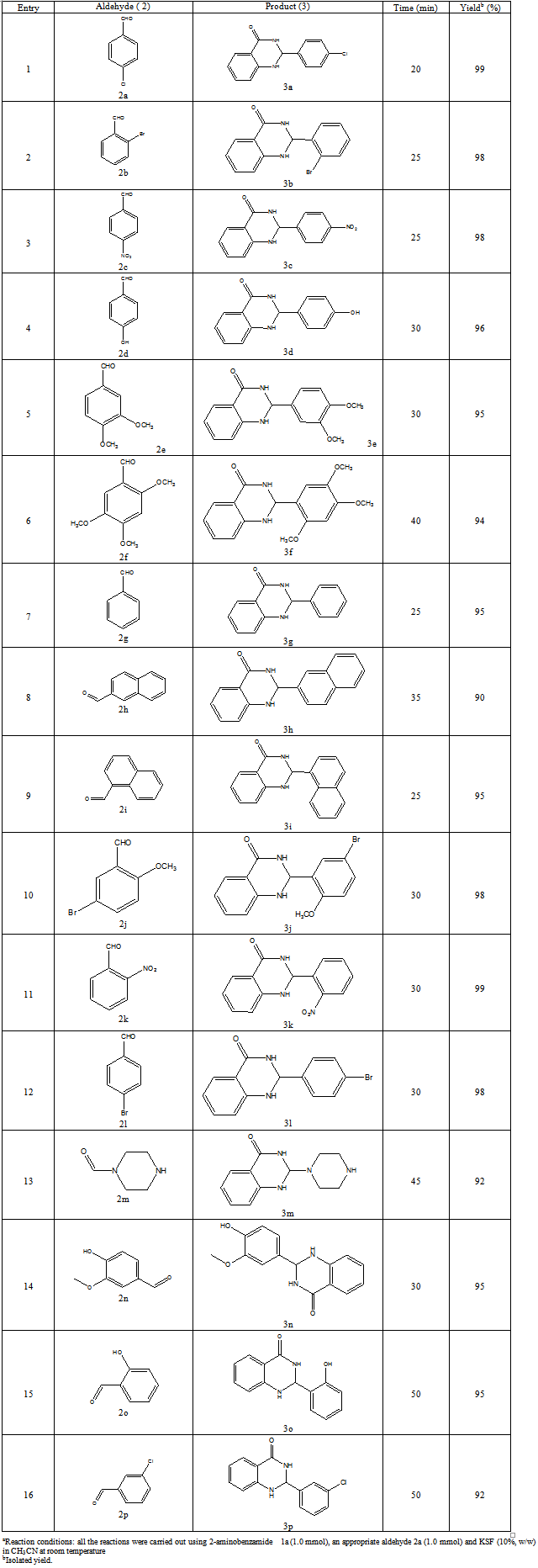
- The evolution of simple and efficient synthetic routes to organic compounds from readily available and proficient reagents is one of the foremost challenges in organic synthesis. The field of heterogeneous catalysis has captivated the interest of researchers working in the field of catalysis due to increasing necessity for more environmentally acceptable processes in the chemical industry focusing largely on chemical yield, eliminating waste at source and circumventing the use of perilous materials (1). In this sense, clay catalyzed organic reactions have many advantages, such as ease of handling, separation, recycling, environmentally safe disposal, easy availability and their low cost. In addition to that clay minerals occur abundantly in nature and their high surface area, sorptive and ion-exchange properties have been exploited for catalytic applications through decades. Therefore, clay as a substitute of homogeneous catalyst or an expensive heterogeneous catalyst seems highly desirable (2).2,3-Dihydroquinazolinones are the group of heterocyclic compounds in which different substitutions can be made to show pharmacological, Biological and Medicinal activity e.g. Antitumor, Antibiotic, Antipyretic, analgesic, antihypertensive, diuretic, Antihistaminic, antidepressant, and vasodilating agent(3). In addition 2,3-dihydro-2-phenylquinazolin-4(1H)-ones are potent tubulin inhibitors with impressive antiprolivative activity against several human cancers with IC50 value in nano molar concentration. It acts analogously to Colchicine (inhibitors of tubulin polymerization) by binding to α, β- tublin (4).The 2,3-disubstituted quinazolones have been predicted to possess antiviral and antihypertensive activities (5). Additionally, these compounds can easily be oxidized to their quinazolin-4(3H)-one analogs, which is defined as a class of molecules that are capable of binding to multiple receptors with high affinity (6). These compounds also prove prospective antiprotozoal, immunosuppressive, and anticancer activities, the latter of which have been studied most frequently.Various synthetic methods have been reported to prepare this class of compound using an assortment of catalysts like Iodine in Ionic Liquids (7), tetra butyl ammonium bromide(8), iridium (9), ionic liquids (10), iodine (11), ammonium chloride (12),[bmim] HSO4 (13), gallium trifluoro methane sulfonate (14), some Bronsted acids (15), TiCl / Zn (16), phosphoric acid (17), citric acid (18), copper chloride (19) and some other catalytic systems like low valent titanium (20) etc. Su W has reported a method for synthesis by reductive cyclisation of o-nitrobenzamide or o-azidobenzamide with aldehydes and ketones promoted by metallic samarium and a catalytic amount of iodine or SmI2 (21). Due to some drawbacks of these procedures such as tedious process, long reaction times, harsh reaction conditions, poor yields along with the use of non-recyclable and toxic catalysts sometimes ineffectiveness associated with most of these catalysts demands development of some greener process involving low cost, easily available, easy to handle and efficient catalysts. We have developed an environmentally benign, high yielding and kinetically fast synthesis of 2, 3-dihydro-2-phenylquinazolin-4(1H)-one by using Anthranilamide with different substituted benzaldehyde by using clay catalyst. It is inexpensive, non-hazardous, recyclable, efficient and easy to separate from the reaction mixture via simple filtration (22). We tried different clays, out of which Montmorilonite KSF shows better activity in terms of high yield with no side products and could be recycled at least six times and the desired product can be isolated in excellent yield in each run .
 | Scheme 1. |
2. Experimentalx
2.1. General Procedure
- Various substituted aldehyde (1 equiv), anthranilamide (1 equiv), catalyst (0.1 equiv) was stirred at room temperature in acetonitrile for a specified period of time. After the completition of the reaction, monitered by the TLC, the catalyst was filtered and washed with ethyl acetate and the resulting filterate was dried and concentrated on rotavapour. The product was purified by crystallization technique using Ethyl acetate and Hexane as two solvent to afford the pure 2, 3-dihydroquinazolin-4(1H)-one. The products thus obtained are characterized by HRMS, NMR. The spectral data were found to be consistent with authentic samples.
3. Result and Discussion
- At the onset of the research, we investigated the model cyclo-condensation reaction between anthranilamdes and 4-chlorobenzaldehyde (Scheme.1) at room temperature and under different reaction media. After screening a variety of reaction media, Acetonitrile was found to be the most effective medium for the generation of the desired product (Table 1, entry 1) with no side products which may be due to the dipole moment (3.92 D) of Acetonitrile. The use of other solvents such as DCM and methanol (Table 1, entry 2and 3) was examined which did not improve the product yield but produce some unidentified by-products. When the reactions were conducted in water, the expected products were obtained in lower yields and with longer reaction times compared to organic solvents used, but still it was better than those earlier reports. (Table 1, entry 6). The lower yield obtained in water medium may be due to the suppression of condensation, following Le-Chatliers principle.
|
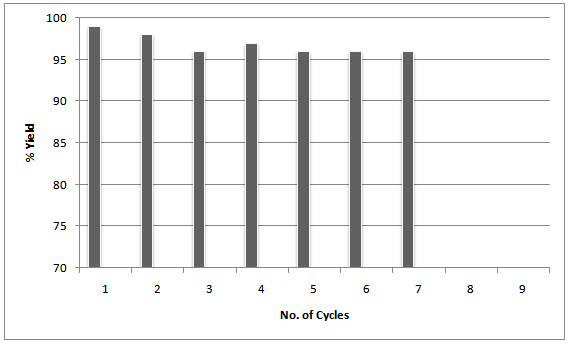 | Figure 1. Recovery cycles of the catalyst |
|
|
4. Conclusions
- In conclusion, we developed a simple and environmentally friendly clay catalysed synthesis of a novel class of quinazolinones. This method allows the use of the inexpensive, easy to prepare, efficient , easy to separate from reaction mixture and reusable clay as the catalysts and offers good results for most 2, 3-dihydroquinazolin-4(1 H)-ones. Starting materials are inexpensive and commercially available. By the reaction of a range of anthranilamides and substituted benzaldehyde, novel libraries of 2, 3-dihydroquinazolin-4(1 H)-ones could be obtained, which would make this method a suitable candidate for combinatorial and parallel synthesis in drug discovery.
ACKNOWLEDGEMENTS
- Authors are grateful to the authorities of M/S Indian Institute of Integrated Medicine (IIIM), canal road, Jammu for providing necessary facilities to carry out the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML