-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(5): 245-247
doi: 10.5923/j.chemistry.20120205.01
Characterization of the Gypsum Composite for Electrical Conductivity
O. Louie 1, A. H. Massoudi 1, M. M. Ejtehadi 2, S. Sajjadifar 1, M. Mirghani 3, S. J. Alavi 1
1Department of Chemistry, University of Payam noor, Mashad, P.O.Box. 91735-433, Iran
2Department of Geology, University of Payam noor, Mahsad, P.O.Box. 91735-433, Iran
3Yazd University - Department of Industrial Engineering, P.O.BOX 89195-741, Iran
Correspondence to: O. Louie , Department of Chemistry, University of Payam noor, Mashad, P.O.Box. 91735-433, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the nature, calcium sulfate is found as gypsum (CaSO₄,2H₂O) in nonconductor compound which converting to plaster by the effect of heat and water loss. Into the autoclave, under pressure Alpha & Beta will be formed of gypsum. In order to create conductivity properties in gypsum, a kind of polymer called poly-aniline has been used which due to environmental sustainability, color changing PH, relatively high electrical conduction and lower price, is preferred by many users. By mixing calcium sulfate with poly-aniline, conductive composite of poly-aniline – gypsum ( PANI – Gypsum ) will be formed. The composite was characterized by FTIR , NMR, UV spectroscopy and standard conductivity measurement. It has been shown that non conductive gypsum could be conducive if it combined with poly-aniline in a poly-aniline – gypsum composite form. By oxidation and reduction, its electrical conductivity could be controlled. Alpha and beta gypsum in vitro condition in combination with the above polymer has produced composite polymer which has electrical conductivity and it can be used in various chemical, military and in particular electronics industries.
Keywords: Poly-aniline, Gypsum, Poly-aniline-gypsum Composite, Electrical Conductivity
Cite this paper: O. Louie , A. H. Massoudi , M. M. Ejtehadi , S. Sajjadifar , M. Mirghani , S. J. Alavi , "Characterization of the Gypsum Composite for Electrical Conductivity", American Journal of Chemistry, Vol. 2 No. 5, 2012, pp. 245-247. doi: 10.5923/j.chemistry.20120205.01.
1. Introduction
- Gypsum (CaSO₄,2H₂O) is a result of calcium sulphate crystallization in monoclinic system with 2.32 of specific gravity and hardness degree 2 which is observed in the nature as spear-shaped crystals, filamentary, masses of fine-grained (alabaster), transparent (selenite) or mixed with clay (gypsyt) and is the most common commercial mineral of calcium sulfate. The mineral takes shape in open and closed basins (intra-continental basins), where the evaporation rate is high, with the most potential energy after the carbonates. If gypsum is heated to 500 º C, gradually loses its water and changes into dead plaster ( CaSO₄.½H₂O).Dead plaster has no ability to absorb water and uses it as a filling material, sometimes used for special cement. In higher temperatures as well, gradually loses all the water and some of its sulfur and converts into hydraulic gypsum and if water be added to it, it will slowly start to firm. Hardness and strength of the resulting object is relatively high.The two Alpha and Beta form of gypsum are formed depending on different pressure and humidity.The beta (β) type of the hemi hydrate gypsum is formed. In vacuum with pressure lower than one atmosphere which due to the low solubility and short setting time it is used as asphalt emulsifier, in vermiculite, wall board, etc. The alpha (α) type of Gypsum is achieved in an autoclave under high pressure and the presence of water vapor. This type of gypsum has gained many importance and applications due to less water absorption and high resistance to exerted stress, and can be used in the manufacture of dental plaster and molding (medical application). Due to its unique properties such as adhesion, volume increase, high water absorption and good mechanical strength, Gypsum has been among the most common applied minerals among which one can point to combining it with Portland cement, fiber glass, plastic resins and other materials to build tension and fire resistant products[1-2]. One of the important physical characteristics of compounds and elements in the nature, is the electrical resistance and their electrical conductivity, because these two properties are inverse to each other[3]. Gypsum, due to ionic bonding between its cationic and anionic radicals of which it is made, is a kind of salt and the absence of free electrons therein has caused poor electrical conductivity of this mineral[4]. Synthetic poly aniline in specific circumstances has sufficient free electrons and according to this reason, it enjoys electrical conductance, thus being named as a conducive polymer[11-16].By mixing poly aniline and gypsum under certain temperature and pressure, novel poly aniline-gypsum composite is produced which has special electric conductivity[17]. The experiments show that electron transfer mechanism within the crystalline network of gypsum is controlled by bands of poly-aniline was shown in “figures 3”[18].Making this composite would task gypsum with a new role in various industries such as electronics, military, and painting.
2. Discussion
- 0.3 gr Potassium iodat (Aldrich, ACS reagent, 99.5%) was dissolved in 50 ml of a one- molar solution of ammonium persulfate ([(NH4)2S2O8], APS).5 ml of aniline (Shengyang Federation Reagent Factory, 99%) was added to a solution and after 2 hours of stirring, the polymer was filtered and then dried.Poly-aniline in two types of gypsum b and a, in an even mass proportion (0.1 gr : 0.1 gr) and under a 10- bar pressure is combined and thus two types of poly-aniline - gypsum composites are obtained.Electrical conductivity of various polymers is measured by the polymer conductivity meter. Table 1, shows the values of electrical conductivity of poly aniline, the produced composites and gypsum.
|
 | Figure 1. Polymer Translocation within the Gypsum Crystal Network |
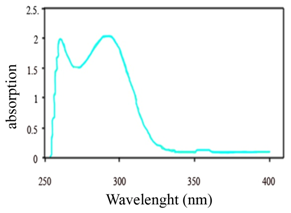 | Figure 2. UV Spectrum of Poly Aniline |
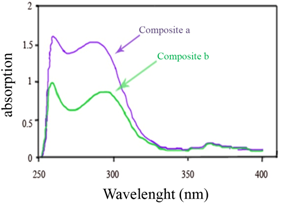 | Figure 3. UV Spectrum of “a” and “b” Composite |
3. Conclusions
- Increasing the electrical conductivity observed in the composite gypsum indicates a change in the delivery of composite gypsum mineral from 56 Siemens to about 3 in the recombinant composite which could be used as a conductor to manufacture electronic parts with specific conductance.Another application of it, is using in military industries especially in anti-radar coatings.In painting industry it also has important application.By use of this, new combination anti-static electricity colors can be produced and in PANI based coatings can prevent corrosion even in scratched areas where bare Steel surface is exposed to the aggressive Environment[19-20].On the other hand it can be used in many applications, such as electromagnetic interference (EMI) shielding, Electro-catalysts, rechargeable battery, chemical sensor, corrosion devices and microwave absorption[21-22].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML