M. J. A. Abualreish 1, 2
1Department of Chemistry, Faculty of Science, Northern Boarders University, Arar, Kingdom of Saudi Arabia (current address)
2Department of Chemistry, Faculty of Science and Technology, Omdurman Islamic University, Sudan (permanent address)
Correspondence to: M. J. A. Abualreish , Department of Chemistry, Faculty of Science, Northern Boarders University, Arar, Kingdom of Saudi Arabia (current address).
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The thermal decomposition of potassium peroxydisulphate at 70℃ under catalyzed conditions was investigated. The decomposition was catalyzed by different systems namely, the silver (I) ion, the copper (II) ion and in the presence of both ions (co- catalysts). It is obvious that the presence of small amount of silver(I) or copper (II) ion enhance the rate of decomposition of peroxydisulphate. A systematic kinetic study showed that the catalytic influence of the different systems used in the decomposition is in the following order :co-catalyst > Ag + > Cu2+ .
Keywords:
Thermal Decomposition, Potassium Peroxydisulphate, Co-catalyst
Cite this paper: M. J. A. Abualreish , The Influences of Catalysts and Co-catalysts on the Thermal Decomposition of Potassium Peroxydisulphate in Aqueous Solution at 70 ℃, American Journal of Chemistry, Vol. 2 No. 4, 2012, pp. 214-217. doi: 10.5923/j.chemistry.20120204.05.
1. Introduction
The thermal decomposition of potassium peroxydisulphate was the subject of study of many workers [1-3], they all suggested that the decomposition follows a first order kinetics. Early workers [4], studying the thermal decomposition of potassium peroxydisulphate found that the decomposition in aqueous solution was accelerated by rise of temperature and depends on the concentration of solution. Levr and Migliorini [5] have found that the thermal decomposition of potassium peroxydisulphate was unimolecular and is catalyzed by acid. The silver ion catalysed decomposition of peroxydisulphate ion was studied by Bawn and Margrerison [6], their studies showed that the decomposition is first order in both silver and peroxydisulphate ion. Abualreish [7] studied the uncatalyzed thermal decomposition of potassium peroxydisulphate. He found that the decomposition is first order in peroxydisulphate concentration and satisfies the relation :R = KO[S2O82-]O the value the observed rate constant ko equal to 0.54 X 10-5 sec-1. Bartlett and Cottman [8] have suggested that the radicals produced in the Peroxydisulphate ion decomposition in aqueous solution cannot induce the decomposition of the ion and that autocatalysis is not observed in the thermal decomposition of peroxydisulphate.
2. Experimental
All chemicals used were AnalaR grade. All solutions were prepared according to the usual analytical procedures. Deionized water was used in all kinetic runs. Since the reaction does not take place to any measurable extent at room temperature (t 1/2 = one month) [9], the temperature used was70 ℃. The iodometric method was used for the analysis and estimation of unreacted peroxydisulphate [S2O82-] which is a modification of the method used by Bartlett and Cottman [8] and Rosin [10]. In this method to 5 ml of the reaction mixture, 5 ml sodium bicarbonate and one ml of sulphuric acid were added to maintain a pH value of 7.1 to 7.2. The liberated iodine was titrated against sodium thiosulphate using starch as indicator.
3. Results and discussion
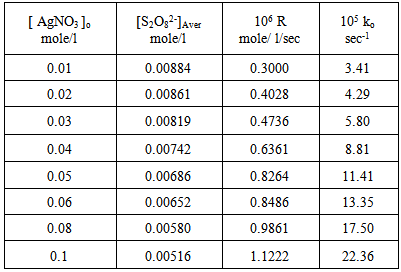
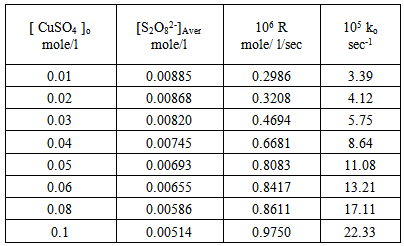
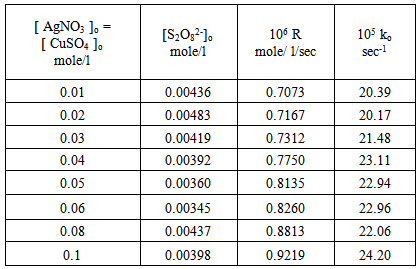
For many reducing agents. oxidation by peroxydisulphate does not proceed at a convenient rate at 25 ℃ , unless a catalyst is present. The most thoroughly investigated catalyst is the silver (I) ion [11], although reactions involving the copper (II) ion [12] and the iron (II) ion [13] also have been studied. Dependence of the rate of decomposition of peroxydisulphate on the systems: [ Ag+ ], [ Cu2+ ] and [Ag+ + Cu2+ ] : Tables (1), (2) and (3) show the catalytic effect of Ag(I), Cu(II) and [Ag(I) + Cu(II) ] ions on the thermal decomposition of potassium peroxydisulphate at 70oC in which the concentration of Ag(I), Cu(II) and [Ag(I) + Cu(II) ] ions varies from 0.01 to 0.1 mole/l while that of peroxydisulphate was kept constant at 0.01 mole/l. Results were represented graphically in figures (1) and (2) for Ag(I) ion, (3) and (4) for Cu(II) ion and (5)and (6) for [Ag(I) + Cu(II) ] ion. Table 1. Dependence of the rate of decomposition of peroxydisulphate on Ag(I) ion
 |
| |
|
Table 2. Dependence of the rate of decomposition of peroxydisulphate on Cu(II) ion
 |
| |
|
Table 3. Dependence of the rate of decomposition of peroxydisulphate on [Ag(I) + Cu(II) ] ion
 |
| |
|
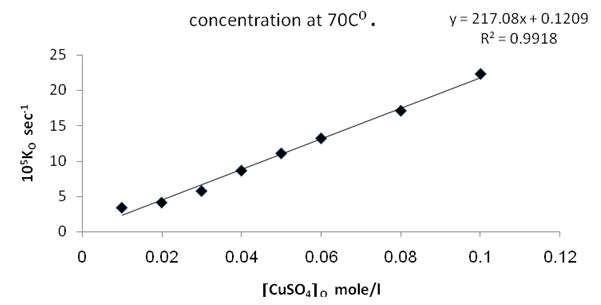
Figures (1) and (3) , show that the value of ko increases linearly with the increasing concentration of Ag(I) and Cu(II) ions respectively , indicating first order dependence of the reaction on the catalyst concentration. In the case of the system [Ag+ + Cu2+ ] (figure (5) ), the value of ko does not increases linearly with the increasing concentration of [ Ag+ + Cu2+ ]. It that means the value of ko is independent of the initial co- catalyst concentration.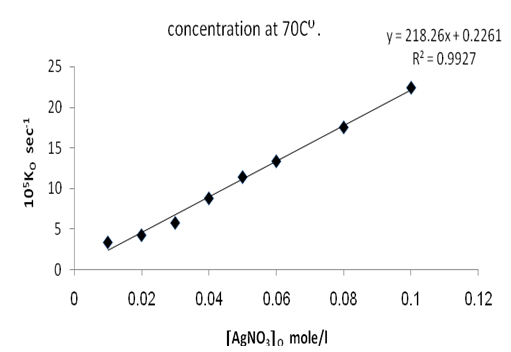 | Figure 1. Observed rate dependence on Ag+ion concentration at 70 ℃ |
 | Figure 3. Observed rate dependence on Cu++ion concentration at 70 ℃ |
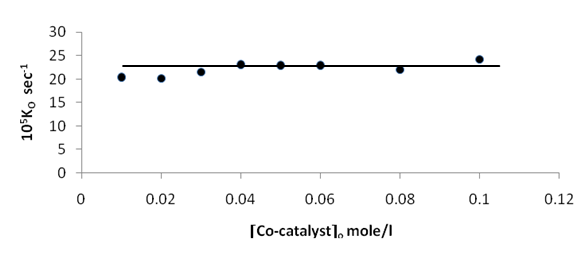 | Figure 5. Plot of the observed rate constant ko against [Co-catalyst]o at 70 ℃ |
From figures (2) , (4) , and (6) the intercept at the rate axis is equal to 0.25X10-6 mole/l/sec for Ag(I), 0.23X10-6 mole/l/sec for Cu(II), and 0.67X10-6 mole/l/sec for [Ag(I) + Cu(II) ] ion. Therefore the observed first order rate law for the three systems can be expressed as follows :R = 0.25X10-6 + ko [Ag+]oR = 0.23X10-6 + ko [Cu2+ ] oR = 0.67X10-6 + ko [Ag + + Cu2+ ]o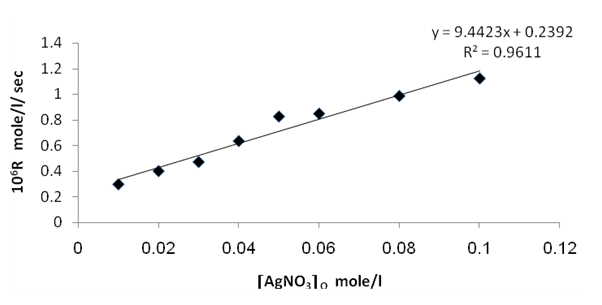 | Figure 2. Rate dependence on Ag+ ion concentration at 70 ℃ |
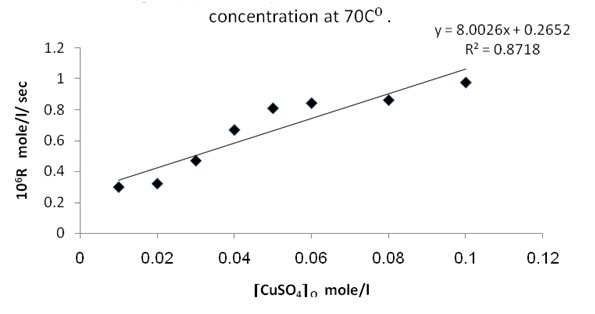 | Figure 4. Rate dependence on Cu++ ion concentration at 70 ℃ |
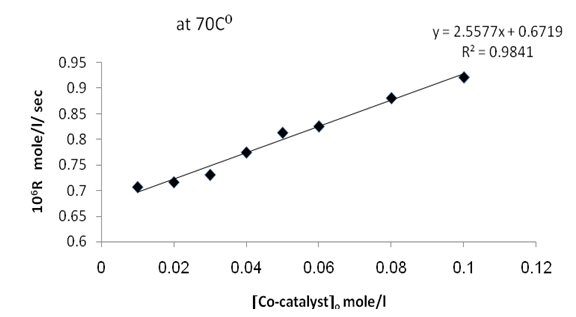 | Figure 6. Plot of the rate R against [Co-catalyst]0 at 70 ℃ |
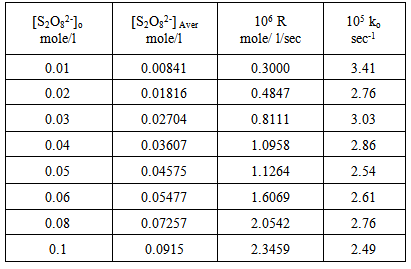
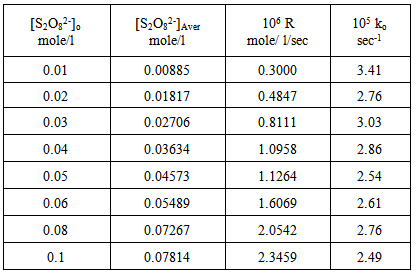
The reactions were also studied at various peroxydisulphate concentrations and constant [Ag+], [Cu2+ ] and [Ag+ + Cu2+] concentrations . Results are shown in tables (4), (5) and (6) and represented graphically in figures (7) and (8) for Ag(I) ion, (9) and (10) for Cu(II) ion and (11) and (12) for [Ag(I) + Cu(II) ] ions.Table 4. Peroxydisulphate dependence : [ AgNO3]o = 0.01mole/l Temp. 70 ℃
 |
| |
|
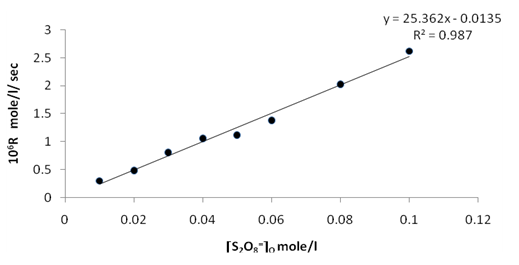
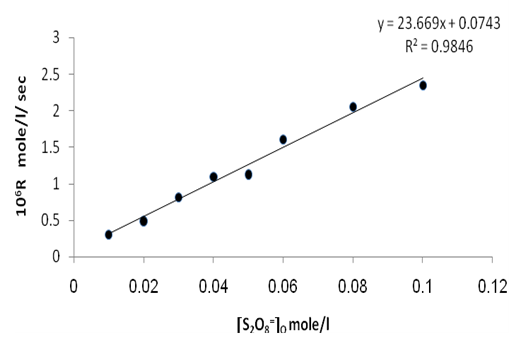 | Figure 7. Rate dependence on [S2O8=]o at 70 ℃ |
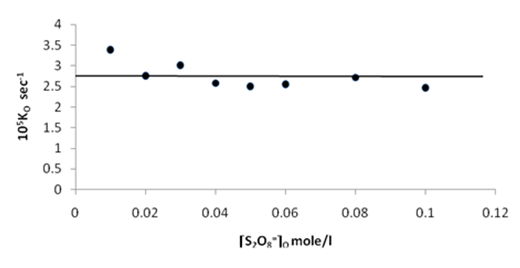
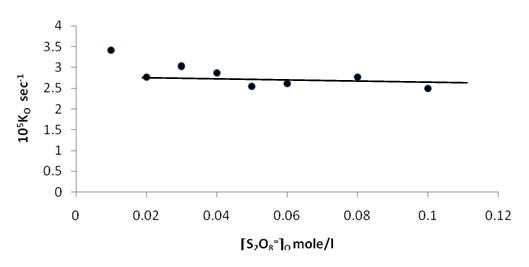 | Figure 8. Plot of the observed rate constant Ko against [S2O8=]o at 70 ℃ |
Table 5. Peroxydisulphate dependence : [ CuSO4 ]o = 0.01mole/l Temp. 70 ℃
 |
| |
|
 | Figure 9. Rate dependence on [S2O8=]o at 70 ℃ |
 | Figure 10. Plot of the observed rate constant Ko against [S2O8=]o at 70 ℃ |
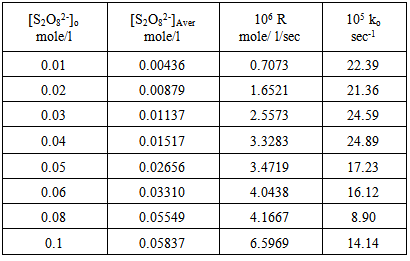
Table 6. Peroxydisulphate dependence : [ AgNO3 ]o = [ CuSO4 ]o = 0.01mole/l Temp. 70 ℃
 |
| |
|
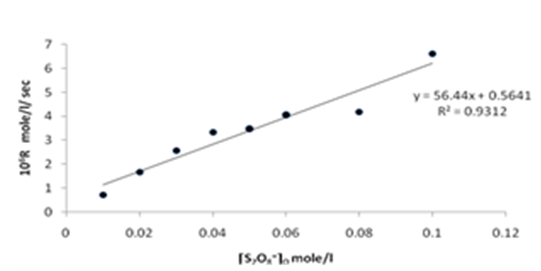 | Figure 11. Rate dependence on [S2O8=]o at 70 ℃ |
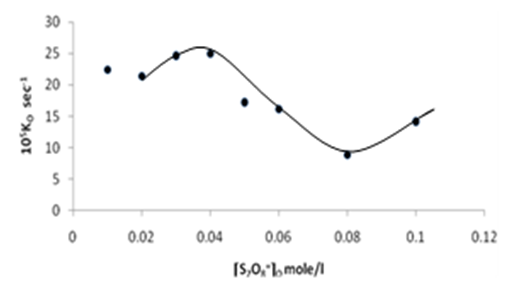 | Figure 12. Plot of the observed rate constant Ko against [S2O8=]o at 70 ℃ |
Figures (7), (9), and (11) show the linearity of the initial peroxydisulphate concentration [S2O82-]o with the rate ( R) for the three systems , so the rate can be expressed by the following equation :R = ko[S2O82-]o. Also, it is obvious from figures (8) and (10) that the relation is linear and parallel to the concentration axis giving a graphical mean of 2.8X10-5 sec-1 in case of Ag+ ion and 2.7X10-5 sec-1 , in case of Cu2+ ion .Therefore we conclude that the values of ko were found to be independent of peroxydisulphate concentration, so the reaction is first order in [Ag+] and [Cu2+] and zero order in [S2O82-]. In the case of the system [Ag+ + Cu2+ ] (figure (12)), the relation is not linear and that means the order of the reaction with respect to peroxydisulphate is unknown.Table 7. [S2O8= ] = [ Ag+] = [Cu++ ] = [ Ag++ Cu++ ] = 0.01mole/l Temp70 ℃
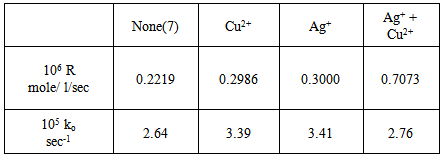 |
| |
|
Table (7) includes a summary of the values of (R) and (ko) for the catalytic and the co-catalytic thermal decomposition of potassium peroxydisulphate at 70 ℃. The above findings showed that the copper ion was much less efficient than the silver ion as catalyst for the thermal decomposition of peroxydisulphate. Finally, we can deduce that the catalytic influence of the different systems used in the thermal decomposition of peroxydisulphate is in the following order: co-catalyst > Ag+ > Cu2+.
References
| [1] | Morgan, J. L, Christ, R. H, (1927). J. Amer. Chem. Soc, 49, 338 – 961. |
| [2] | Fronaeus, S, Ostman, (1955) .C.O, Acta. Chem. Scand, 9, 902. |
| [3] | Kolthoff, I.M, Miller, I.K, (1951). J. Amer.Chem. Soc, 73, 3055 . |
| [4] | Green, L, Mason, D.O, (1910).J. Chem. Soc, 97, 2083. |
| [5] | Levr, M.G, Migliorini, E, and Gazz,A, (1963). Chim Ltal, 36(ii), 599. |
| [6] | Bawn,C. E, Margersion, D,(1955) Trans. Fraday.Soc,51, 925. |
| [7] | Abualreish, M.J, (2008). Material Science Research India, 5(2), 261. |
| [8] | Bartlett, P.D,Cotman, J.D., (1949).J. Amer.Chem. Soc., 71, 1419. |
| [9] | Vasudeva,W.C, Ph.D thesis, (1969). Fac. of Science, U. Of K. |
| [10] | Rosin, J. “Reagents Chemicals and standards, (1946). D. van Nostrand. CO. Inc. New. York, 2nd edition .P. 349. |
| [11] | Chasi,K, etal, (1977) Bull.Chem.Soc. Japan, 50, 3049. |
| [12] | Sethna, S.M, (1951) Chem.Rev, 49, 91. |
| [13] | Benzvi, E, Allen, T.J, (1961) J. Amer.Chem. Soc., 83, 4352. |













 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





