-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
2012; 2(4): 186-190
doi: 10.5923/j.chemistry.20120204.02
Cationic Surfactants from Arginine: Synthesis and Physicochemical Properties
Pravin U. Singare 1, Jyoti D. Mhatre 2
1Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 4000058
2Department of Chemistry, Shri. Jagdishprasad Jhabarmal Tibrewala University, Jhunjhunu, Rajasthan 333001
Correspondence to: Jyoti D. Mhatre , Department of Chemistry, Shri. Jagdishprasad Jhabarmal Tibrewala University, Jhunjhunu, Rajasthan 333001.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present invention concerns the preparation of cationic surfactants derived from the condensation of an acid chloride, preferably a fatty acid with a number of carbon atoms 8, 9 and 14 with esterified amino acids, preferably basic-type amino acids, like (L)-arginine. The method comprises a first step in which the esterification of the amino acid with an alcohol is performed and a second step for the condensation with a chloride of fatty acid, using Schotten Baumann conditions. These surfactants constitute a novel class of chemicals of low toxicity with excellent surface properties and considerable antimicrobial activity. As in a conventional series of surfactants with different chain lengths, changes in the chain result in changes in the physicochemical properties. Excellent antimicrobial activity is observed for the homologue of 14 carbon atoms.
Keywords: Cationic Surfactants, NΑ-Acyl, Arginine, Schotten Baumann
Article Outline
1. Introduction
- Arginine based cationic surfactants are amphiphilic compounds that possess excellent self-assembling properties, a low toxicity profile, high biodegradability and a broad antimicrobial activity, which make them candidates of choice as preservative and antiseptics in pharmaceutical, food and dermatological formulations[1-5].The value of amino acids as raw materials for the preparation of surfactants was recognized as soon as they were discovered some 50 years ago. Initially they were used as preservatives for medical and cosmetic applications and were subsequently found to be active against various disease-causing bacteria, tumors and viruses. There is a large variety of amino acid/peptide structures and the fatty acid chains can vary in their structures, length and number, which explains their wide structural diversity and different physicochemical and biological properties[6]. In the last two decades, a group of scientists has published a number of papers addressing the synthesis and properties of biocompatible cationic amino acid based surfactants of different structures[7-10]. These surfactants show a low toxicity profile and an antimicrobial activity similar to those of conventional cationic surfactants. Lipoaminoacids derived from L-Arginine are a recently described family of nontoxic and biodegradable cationic surfactants with antimicrobial properties[1,3]. Arginine based surfactants constitute a promising alternative to other antimicrobial surfactants with high intrinsic toxicity and questioned biodegradability such as quaternary ammonium halides[11-12]. The antimicrobial activity of the argnine-based cationic surfactants is directly associated with the presence of the cationic charge of the protonated guanidine group of this amino acid[13]. Amino acid based surfactants have some distinctive structural features as shown by general chemical formula of Nα- acyl arginine derivatives, the surfactants object of this study (Fig. 1). (A) The special properties exhibited by these type of compounds are due to the strong hydrogen bonding of the amide bond located between the hydrophilic (amino acid residue) and hydrophobic part of the molecule. (B) Presence of asymmetric carbon atom in the molecule making formation of the chiral aggregates[14].In this paper, the main part of the systematic study whose aim deals with the influence of terminal fatty acid chain on the properties of Nα- acyl arginine esters is reported. Nα- acyl arginine derivatives that contain basic amino acid (Arginine) as terminal amino acid have been prepared by peptide synthesis methods. These compounds have been synthesized as ethyl esters and their fundamental surfactant properties and antimicrobial activities have been evaluated. The properties of these compounds have been compared to the properties of the cationic monomer derivative methyl ester of Nα- lauroyl arginine and the amphoteric monomer derivative Nα- lauroyl arginine reported earlier.In this work, three arginine-derivative surfactants, ethyl esters of Nα- Octanoyl arginine, Nα- Nonanoyl arginine and Nα- Myristoyl arginine are studied. We report the chemical synthesis and the study of some physical properties such as critical micellar concentration. Biological property such as antimicrobial activity is also investigated.
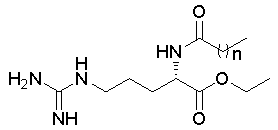 | Figure 1. Molecular structure of the Nα-acyl arginine ethyl ester surfactants; n=6 CAE, n=7 NAE, n=14 MAE |
2. Experimental
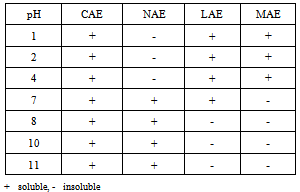
- MATERIALSThe following Nα-acyl arginine monopeptides have been studiedCAE: N-α-Octanoyl-L-Arginine ethyl esterNAE: Nα-Nonanoyl L-Arginine ethyl esterMAE: Nα-Myristoyl-L-Arginine ethyl ester
2.1. General reagents and Synthetic Method-
- L-Arginine was purchased from Ajinomoto Co., Octanoic acid, Nonanoic acid, Myristic acid and Sodium Dodecyl sulfate (SDS) were received from Sigma-Aldrich. LAE.HCl (Nα-Lauroyl arginine ethyl ester Hydrochloride) was supplied by local supplier. General reagents were of an analytical grade and higher purity. Solvents used were of analytical grade or higher purity and supplied by Sigma-Aldrich. All fatty acid chlorides are prepared in our lab. L-Arginine Hydrochloride is prepared following the procedure of literature (18). The homogeneity of compounds was checked by thin-layer chromatography on aluminium plates (Kieselgel G, Merck}. The solvent systems were (A) chloroform/methanol/acetic acid (8.5:10:5); and (B) chloroform/methanol (7:3). Ninhydrin developer solution was used for qualitative analysis of free amino groups.Nuclear Magnetic Resonance (1H NMR) and all the NMR measurements were performed with Bruker, Avance 300 spectrometer model at 300MHz in a 5mm direct probe (BBO BB-1H) using CDCl3 as a solvent. Surface Tension was measured using Stalagmometer with a Wilhelmy plate. Mass Spectroscopy with fast atom bombardment (FAB) was carried out with VG-QUATTRO from Fisons Instrument. Method for synthesis
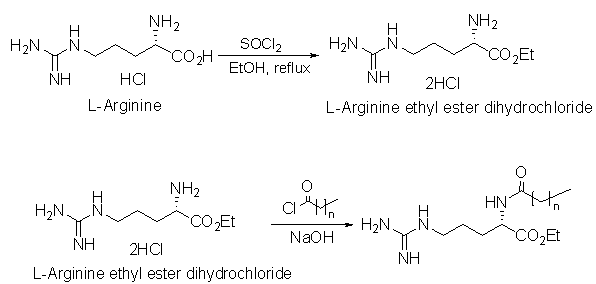 | Figure 2. Schematic method of synthesis |
- Preparation of L-Arginine ethyl ester dihydrochlorideIn a 500ml round bottom flask is charged 250ml Ethyl alcohol followed by the addition of 0.25 equivalent of L-Arginine HCl at room temperature. Thionyl chloride (1.25 equivalents) is then charged slowly controlling exotherm. Heat is applied and reaction mixture is refluxed for 4-5 hours. After completion of the reaction, Ethanol is continuously removed under vacuum, with intermediate additions of dry Ethanol. The residual mass is cooled to get crude L-Arginine ethyl ester dihydrochloride. Preparation of Nα-Acyl L-Arginine ethyl ester compounds by Schotten Baumann reaction.The crude reaction product obtained in the first step is dissolved in water and the pH of the solution is brought to a specific pH value 5.5-7 by the addition of aqueous sodium hydroxide. The pH of the reaction is carefully kept constant at this value until completion of the reaction. To this solution, add 0.96 equivalent of corresponding acid chloride drop-wise, whereby the temperature of the mixture is kept at a temperature of 10-15° C. After completion of the reaction, the stirring is maintained for a further two hours, after which the pH of the solution is adjusted to a final value of 5.5-7 with hydrochloric acid or sodium hydroxide. Finally, the crude reaction product is obtained either by filtration or by distillation.Compound: N-α-Octanoyl-L-Arginine ethyl ester (CAE) – Prepared by reaction between L-Arginine Et ester diHCl and Octanoyl chloride in the presence of aqueous NaOH (Yield 85%). Clear Yellowish oil. Rf: 0.68; MW 328, ESI-MS; m/z 329 (m+H); 1H NMR: δH (CDCl3), 0.89[t, 3H, (CH3 alkyl chain)], 1.29[s, 11H, (4CH2, alkyl chain), (OCH2-CH3)], 1.5-1.7[m, 4H, (-CH2-CH2-CH2-NH-)], 2.048[s, 1H, (-CH2NH-)], 2.21-2.27[t, 2H, (-CH2CO-)], 3.1-3.3[m, 1H, (-CH2NH-)], 3.5-3.7[2H, (-CH2-CO-NH-)], 4.2[m, 2H, (-OCH2-CH3)], 4.44[m, 1H, (-NH-CH-COO-), 4.815[ m, 1H, (-CH2NH-)], 7.24-7.27[m, 2H, (-NH-C(=NH)-NH2)], 8.756[1H, (-NH-CH-COO)]Compound: N-α-Nonanoyl-L-Arginine ethyl ester (NAE)– Prepared by reaction between L-Arginine Et ester diHCl and Nonanoyl chloride in the presence of aqueous NaOH (Yield 80%). Light brown sticky mass. Rf: 0.45; MW 342, ESI-MS; m/z 343 (m+H); 1H NMR: δH (CDCl3), 0.87[t, 3H, (CH3 alkyl chain)], 1.27[s, 10H, 5CH2, alkyl chain], 1.59-1.83[m, 4H, (-CH2-CH2-CH2-NH-)], 2.21-2.27[t, 2H, (-CH2-CO-NH-)], 3.087-3.292[m, 1H, (-CH2NH-)], 3.5-3.7[1H, (-CH2-CO-NH-)] 4.2[m, 1H, (-OCH2-CH3), 4.456[m, 1H, (-NH-CH-COO-), 7.26[3H, (-NH-C(=NH)-NH2)], 8.958[1H, (-NH-CH-COO)]Compound: N-α-Myristoyl-L-Arginine ethyl ester (MAE) – Prepared by reaction between L-Arginine Et ester diHCl and Myristoyl chloride in the presence of aqueous NaOH (Yield 78%). White solidRf: 0.55; MW 412.6, ESI-MS; m/z 413.2 (m+H); 1H NMR: δH (CDCl3), 0.855-0.899[t, 3H, (CH3 alkyl chain)], 1.251-1.299[m, 28H, CH2, OCH2CH3], 1.6-1.9[m, (-CH2-CH2-CH2-NH-)] , 2.26-2.34[t, 2H, (-CH2CO-)], 3.21-3.37[m, 2H, (-CH2NH-)], 4.2[m, 2H, (-OCH2-CH3), 4.43[m, 1H, (-NH-CH-COO-), 7.039[m, 3H, C(=NH)-NH2)], 7.22-7.26[t, 1H, (-CH2NH-)], 7.824[m, 1H, (-NH-CH-COO)].
2.2. Physicochemical Behavior
- To check the behavior of the synthesized monopeptides of arginine as surfactants in solution, the concentration at which the surfactant molecules start to form micelles, known as critical micellar concentration (cmc), was determined. Water/surfactant solutions of different concentrations were prepared and allowed to equilibrate at 25°C between 4 and 10 hr. The conductivity of these aqueous solutions was measured. The conductivity of the aqueous solutions rose linearly with increasing concentrations up to break points that correspond to the cmc of these surfactants. For the sake of comparison, the cmc value of pure commercially available LAE (Nα-Lauroylarginine ethyl ester) was also determined (Table 1). Graphical representation of CMC versus carbon atoms in the hydrophobic chain for Nα-Acyl arginine surfactants,.is shown in Fig. 3.
|
|
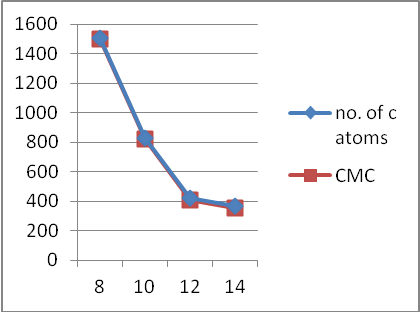 | Figure 3. CMC versus carbon atoms in the hydrophobic chain for the Nα-Acyl arginine surfactants |
2.3. Antimicrobial Activity
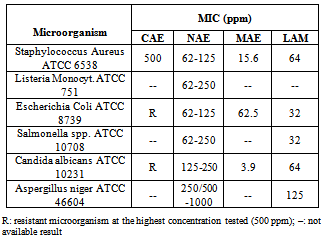
- The microbicidal effects of medium and long-chain fatty acids and their corresponding 1-monoglycerides, of which the most active are the compounds with 12 carbon atoms in the alkyl chain, are well known (10,19). Lauric acid is known to the pharmaceutical industry for its good antimicrobial properties, and the monoglyceride derivative of lauric acid, monolaurin, is known to have more potent antimicrobial properties against enveloped viruses and numerous pathogenic Gram positive bacteria (20). The Antimicrobial activity cannot be determined by any given individual structural moiety alone. It is the right combination of positive charges and hydrophobic groups that provide the adequate hydrophilic-lipophilic balance (21). In order to study the effects of the introduction of the arginine amino acid in these structures on the antimicrobial properties, these compounds were evaluated against Gram positive and Gram negative bacteria. Minimum Inhibitory Concentration (MIC) of molecules is defined as the lowest concentration of antimicrobial agent that inhibits the development of visible micro-organism growth after incubation at 32°C for 48 hrs and fungal growth at 25°C for 4 days by Broth Dilution method. Sample preparation was done by simply mixing 1ml of the 1% solution in DMSO with 9ml of broth Tryptic Soy Broth (1000ppm solution). This stock solution was a clear solution. From the above stock solution 1ml was added to each of 12 consecutive sterile 13mm tubes containing 1ml TSB. Each tube is vortexed and aseptic transfer to give the concentration range of 0.25 to 500ppm. Each culture is grown in TSB>24hrs<48hrs at 32°C. The culture is diluted to 10,000 cfu/ml and 10 μl of this is added to each tube. Negative controls (NC) TSB confirm sterility of the TSB, Positive controls (PC) for each culture confirm organism capable of growth in the TSB. The antimicrobial activity of all synthesized compounds has been established by estimating their corresponding MIC values (in ppm) against Gram-positive and Gram-negative bacteria. For the sake of comparison, the MIC of LAM (Nα-Lauroylarginine methyl ester) has been assessed (see Table 3).
|
2.4. Results and Discussions
- The low surface-tension values of solutions of our acylmonopeptides (27-24 nM/m) and the appearance of a CMC, suggest their utility as surfactants. These values are comparable to the 25.5 nM/m obtained for micellar solutions of commercially available surfactants: LAE.HCl (cationic surfactant). As expected, the cmc decreases when the alkyl chain increases as a consequence of the higher hydrophobic content of the molecule. The most hydrophobic compound (MAE) showed the greatest ability to lower the surface tension and to form micelles.In view of the results of the antimicrobial activity of these compounds, the MAE homologue has a broader spectrum of antimicrobial activity than CAE, NAE and even commercial LAM compounds. Table 3 shows that the effectiveness of inhibiting the growth of bacteria decreases in the order of MAE > LAM > NAE > CAE. This optimum effect MAE homologue can be attributed to the combination of several physicochemical parameters: hydrophobicity, adsorption, cmc and aqueous solubility.
2.5. Conclusions
- The following conclusions may be drawn from the present study: i) Nα-Acylarginine ethyl ester can be synthesized in good yields using Schotten Baumann reaction conditions. ii) The surface activity was increased and the cmc decreased by raising the alkyl chain length and the hydrophobicity of the amino acid residue. iii) Increase in the carbon chain length of acyl group of Nα-Acylarginine ethyl ester improves antimicrobial properties.From this study we could summarize that the introduction of an appropriate long chain Nα-arginine residue (In this case 14 carbon atoms) to the amino function of a amino acid yields an interesting multifunctional compound to be applied as a soft preservative peptidic surfactant in cosmetic, foods and dermopharmaceutical formulations.
ACKNOWLEDGEMENTS
- We are indebted to Dr. Vilas Chopdekar and Dr. Richard Stockel for technical support to this project. We are also thankful to V & V Pharma Industries for providing Laboratory to conduct experiments.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML