-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
2012; 2(4): 181-185
doi: 10.5923/j.chemistry.20120204.01
Synthesis and Characterization of Transition Metal Complexes of Chlorpromazine
Yakubreddy Naini 1, Tarab J. Ahmad 1, Gilles K. Kouassi 1, S. Ananda 2, Netkal M. Made Gowda 1
1Department of Chemistry, Western Illinois University, One University Circle, Macomb, 61455, USA
2Department of Studies in Chemistry, University of Mysore, Manasagangothri, Mysore 570 006, India
Correspondence to: Netkal M. Made Gowda , Department of Chemistry, Western Illinois University, One University Circle, Macomb, 61455, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
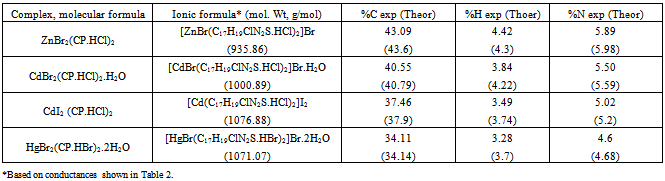
The chlorpromazine (CP) complexes of the transition metal ions, Zn(II), Cd(II) and Hg(II), have been synthesized. The complexes have been characterized by their elemental analysis, molar conductivity, magnetic susceptibility, UV-Visible, IR and 1H-NMR data. The molecular formulations of the new mononuclear complexes have been found to be [ZnBr(C17H19ClN2S.HCl)2]Br, [CdBr(C17H19ClN2S.HCl)2]Br.H2O, [Cd(C17H19ClN2S.HCl)2]I2 and[HgBr((C17H19ClN2S.HBr)2]Br.2H2O, where the ligand chlorpromazine or CP = C17H19ClN2S. The complexes, [ZnBr(C17H19ClN2S.HCl)2]Br, [CdBr(C17H19ClN2S.HCl)2]Br.H2O, and [HgBr((C17H19ClN2S.HBr)2]Br.2H2O, behave in DMF solutions as 1:1 electrolytes while the other complex, [Cd(C17H19ClN2S.HCl)2]I2, shows an ionic ratio of 1:2 in solution. Molecular structures have been proposed showing a square pyramidal environment around each metal center with an sp3d hybridization for the five-coordinate complexes, [ZnBr(C17H19ClN2S.HCl)2]Br, [CdBr(C17H19ClN2S.HCl)2]Br.H2O, and[HgBr((C17H19ClN2S.HBr)2]Br.2H2O. In the four-coordinate [Cd(C17H19ClN2S.HCl)2]I2 complex, the Cd(II) center with an sp3 hybridization has a tetrahedral environment around it.
Keywords: Chlorpromazine, Metal Complexes, Synthesis, Characterization, Analysis
Article Outline
1. Introduction
- The N-alkylamino phenothiazine derivatives (NPTZs) including chlorpromazine (CP) are biologically active heterocyclic compounds. Structurally, CP is a phenothiazine substituted with chlorine and tert-alkylamine groups at 2 and 10 positions, respectively (Fig. 1). The NPTZ derivatives find extensive applications in the field of medicine as antipsychotic, anxiolytic, antiemetic and inodilationdrugs[1-3].
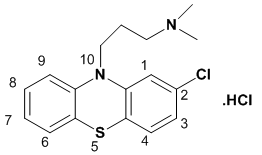 | Figure 1. Molecular structure of chlorpromazine hydrochloride |
2. Experimental
2.1. Materials
- Metal salts, zinc bromide, cadmium bromide, cadmium iodide and mercuric bromide, and the ligand, chlorpromazine hydrochloride (CP·HCl; 99% pure) (Aldrich/Sigma Chemical Company) were used as supplied.All solvents such as methanol, ethanol, diethyl ether, dimethyl sulfoxide, dimethyl formamide and DMSO-d6 (Cambridge isotope laboratories Inc.) were of ACS reagent grade and were used without further purification. Double distilled water was used in all preparations.
2.2. Physical Measurements
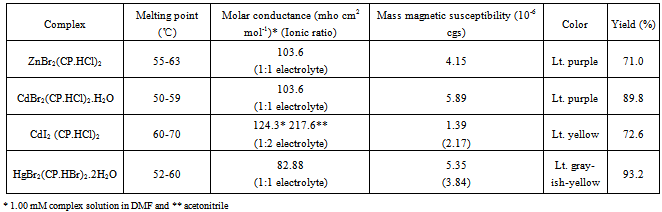
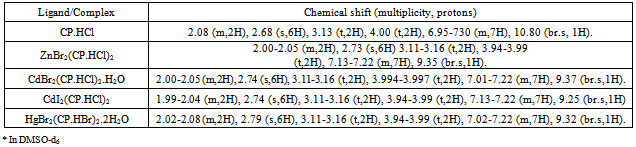
- Elemental analyses of complexes were performed by Microanalysis Laboratory, University of Illinois, Urbana-Champaign, IL. Molar conductance was determined with the Conductance-Resistance meter. UV-Visible spectra were recorded on a Shimadzu UV1601 spectrophotometer. The infrared spectra were recorded on a Shimadzu FTIR 8400 spectrometer using potassium bromide pellets. 1H-NMR spectra were recorded on a JEOL-300MHz FT-NMR spectrometer in DMSO-d6. Mass magnetic susceptibilities of the complexes were measured at room temperature with a Johnson Matthey magnetic susceptibility balance which uses HgCo(SCN)4 as a calibrant.
2.3. General Synthesis of Complexes
- A solution of the transition metal salt (x mmol) (ZnBr2, CdBr2, CdI2 and HgBr2) dissolved in a minimum volume of MeOH was slowly added with stirring to a concentrated methanolic solution of CP.HCl (2x mmol) and refluxed overnight. Each reaction mixture was cooled overnight at 0℃ and the precipitated product isolated by suction filtration through a medium-glass fritted funnel. The product was washed with small amounts of cold water first followed by methanol, air-dried, and dried in vacuo over anhydrous CaSO4 in a desiccator. Each crude product was recrystallized twice from a hot saturated solution of the crude sample in methanol. The yield was determined.
3. Results and Discussion
- The elemental analysis data listed in Table 1 show that the theoretical values are in agreement with the experimental ones. Physical properties of the new metal-CP.HCl complexes are presented in Table 2. Complexes are colored, microcrystalline, and relatively stable at room temperature with percent yields ranging from 71 to 93. The complexes are slightly soluble in common polar solvents such as MeOH and readily soluble in DMF and DMSO. All complexes except Zn(II) complex are insoluble in water.The stoichiometric reactions involved in the complex formation are represented by the equations (1) and (2) below.
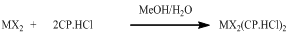 | (1) |
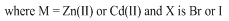
 | (2) |
|
|
|
|
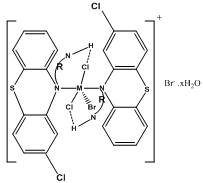 | Figure 2a. Proposed square pyramidal structure for the complex ion, [MBr(CP.HCl)2]+, where R = -C3H6, M = Zn(II) or Cd(II) and x = 0 for Zn(II) and 1 for Cd(II) |
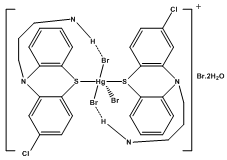 | Figure 2b. Proposed square pyramidal structure for the complex ion, [HgBr(CP.HBr)2]+ |
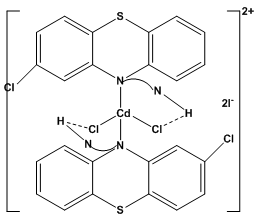 | Figure 2c. Proposed tetrahedral structure for the complex ion, [Cd(CP.HCl)2]2+ |
4. Conclusions
- Four transition metal-chlorpromazine complexes have been successfully prepared and characterized based on their spectroscopic data. Square-pyramidal and tetrahedral geometries have been proposed for the new complexes. The future work would be on the determination of antioxidant and free radical scavenging activities of these complexes using standard assays.
ACKNOWLEDGEMENTS
- The authors are grateful to the Western Illinois University Research Council and the National Cancer Institute-NIH (AREA grant # 1R15 CA115404-01) for support.
References
| [1] | Snyder, S.H., 1976, Amer. J. Psychiatry, 133, 197. |
| [2] | O. Bratfos and J.O. Haug, Acta Psychiat, Scand., 60, 1, 1979. |
| [3] | A.R. Katritzky and A.J. Boulton (Eds.), Advances in heterocyclic Chemistry, Academic press, New York, 1968. |
| [4] | Keshavan, B., and Seetharamappa, J., 1987, Polyhedron, 6(3), 465. |
| [5] | Keshavan, B., and Seetharamappa, J., 1986, Synth. React. Met.-Org. Chem., 16(7), 979. |
| [6] | Keshavan, B., and Janardhan, R., 1987, Ind. J. Chem., 26A, 975. |
| [7] | Keshavan, B., and Janardhan, R., 1987, Ind. J. Chem., 25A, 1054. |
| [8] | Sanke Gowda, H., and Jayarama., 1981, J. Inorg. Nucl. Chem., 43(10), 2329. |
| [9] | Kroener, R., Heeg, M. J., and Deutsch, E., 1988, Inorg. Chem., 27, 558. |
| [10] | Made Gowda, N.M., and Phyu, H.P., 1992, Ttransition Met. Chem., 17, 467; H.P. Phyu, M.S. Thesis, Western Illinois University, Macomb, USA, May, 1991. |
| [11] | Made Gowda, N.M., Phyu, H.P., and Ackerson, B.E., 1993, Transition Met. Chem., 18, 64. |
| [12] | Made Gowda, N.M., Kyi, M.M., and Zhang, L., 1993, Transition Met.Chem., 18, 518; M.M. Kyi, MS. Thesis , Western Illinois University, Macomb, USA, December 1991; Made Gowda, N.M., and Zhang, L., 1994, Synth. React. Inorg. Met-Org. Chem., 24(5), 831; L. Zhang, M.S. Thesis, Western Illinois University, Macomb, USA, May, 1992. |
| [13] | Made Gowda, N.M., Ackerson, B.E., Morland, M., and Rangappa, K.S., 1993, Transition Met. Chem., 18, 271. |
| [14] | Made Gowda, N.M., Pacquette, H.L., Kim, D.H., and Jayaram, B., 1996, J. Mol. Struct., 382 ,129; Made Gowda, N.M., Vallabhaneni, R.K., Gajula, I., and AAFZAL, D., 1996, Synth. React Inorg. Met-Org. Chem, 26(4), 685. |
| [15] | Made Gowda, N.M., Lawrence Pacquette, H., Kim Doo-Hyung, Jayaram, Beby, 1996, J. Mol. Struct, 382, 129-135; Made Gowda, N.M., Rouch, W.D., and Viet, A.Q., 1993, The Chemistry of copper and Zinc Triads., Royal Society of Chemistry, Cambridge, U.K, 117-120. |
| [16] | Made Gowda, N.M., Vallabhaneni, R.K., Gajula, I., Ananda, S., 1997, J. Mol. Struct., 407, 125-130. |
| [17] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2011, Synthesis, characterization, and antioxidant activity evaluation of pyridoxine and its transition metal complexes., Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 41, 1-12. |
| [18] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2009, Synthesis, Characterization and Antioxidant Activity of Zinc(II) and Ruthenium(III) Pyridoxine Complexes., Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 39(8), 434-440. |
| [19] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2010, Synthesis of Iron-Pyridoxine Complex by Solvothermal Process, Its Structural Characterization and Antioxidant Activity Evaluation., J. Chem. Chemical. Engg, 4(12), 33-37. |
| [20] | J.M. Huheey, E.A. Keiter and R.L. Keiter, Inorganic Chemistry; Principles of Structures and Reactivity, 4th ed., Harper Collins College Publishers, 1993. |
| [21] | L.J. Bellemy, The Infrared Spectra of Complex Molecules, Methuen, London, p.355, 1964. |
| [22] | K. Nkamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley Interscience, New York, 1970. |
| [23] | D.A. Skoog and D.M. West, Principles of Instrumental Analysis, Saunders College, P.A, 1980, 171-173. |
| [24] | Jayarama, Thimmaiah, K.N., and D’Souza, M.V., 1985, J. Indian Chem. Soc., 62, 418. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML