-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(3): 171-180
doi: 10.5923/j.chemistry.20120203.13
Sediment Heavy Metal Contaminants in Vasai Creek Of Mumbai: Pollution Impacts
Pravin U. Singare, Manisha P. Trivedi, Ravindra M.
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India
Correspondence to: Pravin U. Singare, Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the present investigation, quantification study of different toxic heavy metals in the sediment samples of Vasai Creek of Mumbai was performed using Atomic Absorption Spectroscopy technique. The study is of importance because such assessment of heavy metals in sediments helps to understand long term pollution load in aquatic environment. Such study will also help to provide a means for evaluating the long term accumulation of heavy metal contaminants. The results of the study indicates that the concentration level of most of the toxic heavy metals like Al, As, Cd, Cr, Hg, Ni, Pb, Sr and Mn for the assessment year 2010-11 were higher than that obtained for the year 2009-10 by the factor of 1.0 to 2.5 µg/g. It is expected that results of present investigation will help in rational planning of pollution control strategies so as to reduce the toxicity impact on marine life of Vasai Creek.
Keywords: Toxic Heavy Metals, Sediments; Marine Life, Quantification, Atomic Absorption Spectroscopy, Cold Vapour Technique, Vasai Creek, Mumbai, India
Article Outline
1. Introduction
- Heavy metals are among the most common environmental pollutants, and their occurrence in waters and biota indicate the presence of natural or anthropogenic sources. Their accumulation and distribution in soil, water and environment are increasing at an alarming rate causing deposition and sedimentation in water reservoirs and affecting aquatic organisms as well[1-5]. Heavy metals like chromium, lead, cadmium, arsenic, etc. exhibit extreme toxicity even at trace levels. Creeks and Rivers are dominant pathways for metals transport[6,7] and heavy metals become significant pollutants of these water bodies[8]. These toxic metals not only pollute the aquatic systems but also pose a threat to the aquatic biota. The increase in residue levels of heavy metal content in water, sediments and biota, will result in decreased productivity [9] and increase in health risk in case of human beings[10-13]. The behaviour of metals in natural waters is a function of the substrate sediment composition, the suspended sediment composition, and the water chemistry. During their transport, the heavy metals undergo numerous changes in their speciation due to dissolution, precipitation, sorption and complexation phenomena[8,14,15] which affect their behaviour and bioavailability[16,17]. For better understanding of heavy metal sources, their accumulation in the sediment and in water seem to be particularly important issues of present day research on risk assessments.In hydrosphere, toxic metal concentrations are typically orders of magnitude greater in the sediments as compared to those in overlying waters. The capacity of sediments to concentrate trace levels of most of the metals make them useful indicators for monitoring purposes and for detecting sources of pollution in the aquatic system. These heavy metals are sensitive indicators for monitoring changes in the water environment. Also the experimental data obtained based on analysis of sediment cores helps to provide a historical record of the heavy metal burdens.The problem of environmental pollution due to heavy metals has begun to cause concern now in most of the major metropolitan cities in Maharashtra state and Mumbai is not an exception to it. The day by day increasing tremendous industrial pollution [18-27] has prompted us to carry the systematic and detail study of pollution due to toxic heavy metals in sediments of Vasai Creek which due to rapid urbanisation and industrialisation is considered as one of the highly polluted creeks of Mumbai.
2. Materials and Methods
2.1. Area of Study
- Vasai Creek is an estuarine creek, one of the two main distributaries of the Ulhas Creek in Maharashtra state of western India. The Ulhas Creek splits at the northeast corner of Salsette Island into its two main distributaries, Vasai Creek and Thane Creek. Vasai Creek which lies between latitude 19.315°N longitude 72.875°E, forms the northern boundary of Salsette Island, and empties west into the Arabian Sea. The Creek receives domestic raw sewage as well as industrial waste water effluent from surrounding habitation and nearby industrial belt. The activities like cattle washing, cloth washing, and religious activities like immersion of idols of Lord Ganesha and Deity Durga during Ganesh festival and Navratri festival is also a major source of pollution of creek water.
2.2. Climatic Conditions
- Climate is subtropical, with mild winters and warm summers. The weather is typical coastal sultry and humid. The average rainfall of records from 1500 mm to 2000 mm. The place experiences the onset of the monsoon in the month of June and experiences monsoon till the end of September. The average temperature recorded in varies from 25 to 37 degrees.
2.3. Requirements
- The chemicals and reagent used for analysis were of analytical reagent grade. The procedure for calculating the different parameters were conducted in the laboratory. The laboratory apparatus were soaked in nitric acid before analysis and then rinsed thoroughly with tap water and deionised distilled water to ensure any traces of cleaning reagents were removed. Finally, it is dried and stored in a clean place. The pipettes and burette were rinsed with the same solution before final use.
2.4. Sediment Sampling and Preparation
- The sediment samples were collected randomly four times in a month in morning, afternoon and evening session at four different sampling stations namely Vasai Bundar (S-1) , Bhayandar west side of Railway Bridge (S-2), Bhayandar east side near Reti Bundar (S-3), and Ghodbundar site (S-4) along the Vasai Creek (Figure 1). The samples were collected and subsequently analysed for a span of two years starting from October 2009 to September 2011. The sampling was done in three shifts i.e. morning shift between 07:00 a.m. to 09:00 a.m., afternoon shift between 02:00 p.m. to 04:00 p.m. and evening shift between 07:00 p.m. to 09:00 p.m. Sediment samples were collected by hand-pushing plastic core tubes (7 cm diameter) as far as possible into the sediment. The sediment cores retrieved in the field were sliced on arrival at the lab at 1-cm depth intervals for the first 15 cm, 2-cm depth intervals from 15–25 cm, and then every 5 cm for the deeper sections of the cores. The sediments were kept cool in icebox during the transportation to the laboratory [28]. They were then ground manually to a fine powder in an alumina mortar; it is passed through a 2-mm mesh screen and stored in polyethylene bags and kept in a dry place until analyses. Well-mixed samples of 2 g each were taken in 250 mL glass beakers and digested with 8 mL of aqua regia on a sand bath for 2 h. After evaporation to near dryness, the samples where dissolved with 10 mL of 2% nitric acid, filtered through Whatman’s No. 1 filter paper and then diluted with deionised water to give final volumes depending on the suspected level of the metals [29]. The samples were subjected to nitric acid digestion using the microwave-assisted technique, setting pressure at 30 bar and power at 700 watts [30, 31]. About 400 mL of the sample was transformed into clean glass separating funnel in which 10 mL of 2% ammonium pyrrolidine dithiocarbamate, 4 mL of 0.5 M HCl and 10 mL of methyl isobutyl ketone (MIBK) are added [32]. The solution in separating funnel was shaken vigorously for 2 min and was left undisturbed for the phases to separate. The MIBK extract containing the desired metals was then diluted to give final volumes depending on the suspected level of the metals [33]. The sample solution was then aspirated into air acetylene flame in an atomic absorption spectrophotometer.
 | Figure 1. Map Showing Sampling Stations along Vasai Creek of Mumbai |
2.5. Heavy metal analysis by AAS technique
- The analysis for the majority of the trace metals like Aluminum (Al), cadmium (Cd), chromium (Cr), nickel (Ni), lead (Pb), strontium (Sr) and manganese (Mn) was done by Perkin Elmer ASS-280 Flame Atomic Absorption Spectrophotometer. Arsenic (As) was determined by hydride generation coupled with an atomic fluorescence detector, while mercury (Hg) was analysed with a cold-vapour atomic adsorption spectrophotometer. The calibration curves were prepared separately for all the metals by running different concentrations of standard solutions. A reagent blank sample was taken through the method, analyzed and subtracted from the samples to correct for reagent impurities and other sources of errors from the environment. Average values of three replicates were taken for each determination.
2.6. Quality Control/Assurance
- Sediment samples were collected with plastic-made implements to avoid contamination. Samples were kept in polythene bags that were free from heavy metals and organics and well covered while transporting from field to the laboratory to avoid contamination from the environment. All reagents were standardised against primary standards to determine their actual concentrations. All instruments used were calibrated before use. Tools and work surfaces were carefully cleaned for each sample during grinding to avoid cross contamination. Replicate samples were analysed to check precision of the analytical method and instrument. To validate the analytical procedures used, the spike recovery test was conducted on some samples for Al, As, Cd, Cr, Ni, Pb, Sr, Mn and Hg.
3. Results and Discussion
- Heavy metals, known to be potentially hazardous substances, are present in both natural and contaminated environments. In natural environments, they occur at low concentrations. However, at high concentrations as is the case in contaminated environments, they result in public health impacts. The elements that are of concern include nickel, cadmium, chromium, mercury, lead, strontium, manganese, Aluminum and arsenic. These toxic heavy metals entering in aquatic environment are adsorbed onto particulate matter, although they can form free metal ions and soluble complexes that are available for uptake by biological organisms. The metals associated with particulate material are also available for biological uptake, and are deposited in estuarine sediments[34]. Once deposited, binding by sulfides and/or iron hydroxides immobilises trace metals until a change in redox or pH occurs[35]. Thus, surfical sediments, particularly the fine fraction, accumulate trace metals and provide a means for evaluating the long term accumulation of heavy metal contaminants[36]. The study of such trace and toxic heavy metals in the environment is more important in comparison to other pollutants due to their non-bio- degradable nature, accumulative properties and long biological half lives. These toxic heavy metals can be absorbed by green plants, which are primary producers in the ecosystem. As they move up the food chain from producers to consumers, they endanger the public health by bioaccumulating in the plant and animal tissues and can cause physiological and neurological disordersThe experimental data on concentration (µg/g) of toxic heavy metals like Al, As, Cd, Cr, Hg, Ni, Pb, Sr and Mn in the sediment samples collected along sampling stations S1, S2, S3 and S4 of Vasai Creek is presented in Table 1. The trend in average concentration of these metals at different sampling stations for two assessment years 2009-10 and 2010-11 is graphically represented in Figures 2-5. From the results it was observed that Aluminum (Al) concentration at different sampling stations lies in the range of 84-278,101-932, 74-448 and 35-398 µg/g at the respective sampling stations. The biyearly average Al concentration was found to be 178, 562, 219 and 226 µg/g respectively at different sampling stations (Table 1). It was also observed that the average Al concentration for assessment year 2010-11 was higher than that obtained for the assessment year 2009-10 by a factor of 1.03 at S2 to 1.68 at S1 (Figures 2-5). It is important here to note that Al toxicity is a potential growth-limiting factor for plants grown in acid soils in many parts of the world [37]. The major Al toxicity symptom observed in plants is inhibition of root growth [38]. The roots exhibit greater signs of cellular damage than other parts of the plant [39]. Al toxicity could be observed in the root system particularly in root-tips and in lateral roots; lateral roots become thickened and turn brown [40]. The root system as a whole is corraloid in appearance with many stubby lateral roots but lacks fine branching. Aluminum interferes with cell division in roots, decreases root respiration and uptake and use of water and nutrients, particularly calcium and phosphorous and metabolic pathway [41].Levels of arsenic (As) are higher in the aquatic environment than in most areas as it is fairly water-soluble and may be washed out of arsenic bearing rocks [42]. Recently, the anthropogenic activities such as treatment of agricultural land with arsenical pesticides, treating of wood using chromated copper arsenate, burning of coal in thermal plants power stations and the operations of gold-mining have increased the environmental pervasiveness of As and its rate of discharge into freshwater habitat [43]. As can also interfere with the fish immune system by suppressing antibody production [44] as well as by lowering macrophage activity and maturation [45]. Several studies are reporting As induced liver fibrosis, hepatocellular damage, inflammation, focal necrosis in addition to hepatocellular carcinoma [46, 47]. In the present investigation it was observed that As concentration at S1, S2, S3 and S4 sampling stations lies in the range of 23-175, 71-429, 82-279 and 61-308 µg/g respectively. The biyearly average As concentration was found to be 112, 285, 159 and 163 µg/g respectively at different sampling stations (Table 1). It was also observed that the average As concentration for assessment year 2010-11 was higher than that obtained for the assessment year 2009-10 by a factor of 1.16 at S3 to 1.42 at S4 (Figures 2-5).
|
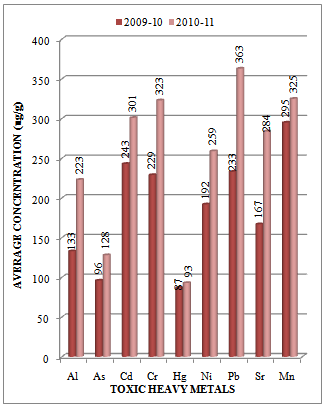 | Figure 2. Variation in average concentration values of different toxic heavy metals in sediment samples collected at S-1 sampling station of Vasai Creek during the assessment year 2009-10 and 2010-1 |
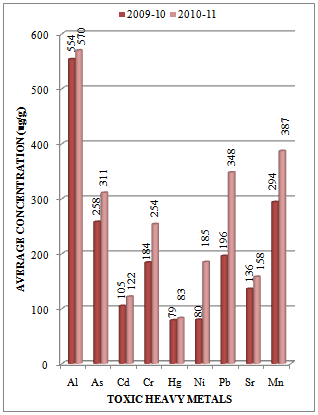 | Figure 3. Variation in average concentration values of different toxic heavy metals in sediment samples collected at S-2 sampling station of Vasai Creek during the assessment year 2009-10 and 2010-11 |
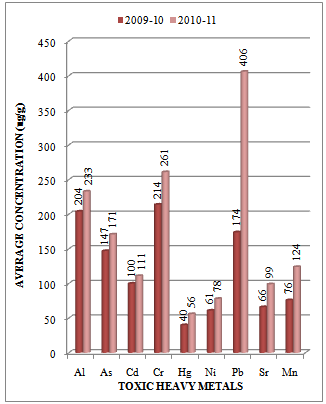 | Figure 4. Variation in average concentration values of different toxic heavy metals in sediment samples collected at S-3 sampling station of Vasai Creek during the assessment year 2009-10 and 2010-11 |
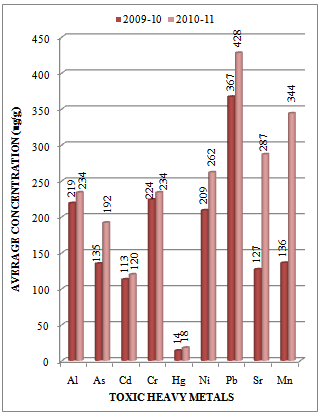 | Figure 5. Variation in average concentration values of different toxic heavy metals in sediment samples collected at S-4 sampling station of Vasai Creek during the assessment year 2009-10 and 2010-11 |
4. Conclusions
- Although in India the Central Pollution Control Board (CPCB) is responsible for restoration and maintaining the wholesomeness of aquatic resources under Water Prevention and Control of Pollution Act 1974 passed by Indian Parliament, it is expected that to maintained or restored the quality of aquatic resources at desired level it is important to have monitoring on regular basis. Although much work has been done on pollution of aquatic resources due to toxic heavy metals, there is still a great need for information on influences of metals and their toxicities fully. The real problem today is not whether heavy metals are toxic or not, since we know that they are: but what concentrations are permissible/safe levels in our waters which do not produce harmful effects on users of water and biological life from the waters. Although it is impossible to prevent pollution of aquatic environment totally, but metal pollution and toxicity could be minimized by certain precautionary measures like development of adequate environmental control and management programmes and by continuous scientific monitoring of our aquatic environment. The present study on monitoring of toxic heavy metals in sediments will help to provide a means for evaluating the long term accumulation of heavy metal contaminants. The present study on pollution due to toxic heavy metals in the sediment samples collected along the Vasai Creek of Mumbai will be useful for rational planning of pollution control strategies and their prioritisation; to assess the nature and extent of pollution control needed and to evaluate effectiveness of pollution control measures already is existence.
ACKNOWLEDGEMENTS
- The authors are thankful to SAP Productions for developing and maintaining the paper template.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML