-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(3): 165-170
doi: 10.5923/j.chemistry.20120203.12
Sorption Study of Co (II), Cu(II) and Pb(II) ions Removal from Aqueous Solution by Adsorption on Flamboyant Flower (Delonix Regia)
Jimoh T. O., Iyaka Y. A, Nubaye M. M
Department of Chemistry, Federal University of Technology, P.M.B.65, Minna, Niger State, Nigeria
Correspondence to: Jimoh T. O., Department of Chemistry, Federal University of Technology, P.M.B.65, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The ability of Delonix regia (Flamboyant) flower to remove Co(II), Cu(II) and Pb(II) ions from aqueous solutions through biosorption was investigated in multimetal batch experiments at 32ºC. The metal ions concentration was determined by atomic absorption spectroscopic (AAS) method. The influence of pH, Contact time, adsorbent dosage and initial metal ion concentration were investigated. The study revealed that maximum removal of Co(II), Cu(II) Pb(II) ion from aqueous solution occurred at pH of 5. The contact time for the adsorption process was found to be at 60 minutes. The amount of metal ions adsorbed increases with increase in adsorbent dosage and initial metal ion concentration. The biosorption of Pb(II) and Co(II) ions exhibited pseudo-second-order kinetics models whereas Cu(II) ion followed for both pseudo first order and second order kinetics model. This study shows that Delonix regia flower is a viable agricultural waste for the removal of Co(II), Pb(II) and Cu(II) ions from aqueous solution.
Keywords: Sorption, Flamboyant Flower, Aqueous Solution
Article Outline
1. Introduction
- The contamination of water by toxic heavy metals is a worldwide problem [1]. This fear has been heightened in recent times due to advancement in technology coupled with increasing industrial activities, both contributed to release of heavy metals into the environment [1-3]. Heavy metals like cobalt, lead, copper, cadmium are present in the environment. They do not degrade or destroy and so, pollute the environment [4]. Uncontrollable discharge of these heavy metals to the environment can be detrimental to humans, animals and plants [5]. These heavy metal ions cause major effects like headache, dizziness, nausea and vomiting, chest pain, tightness of chest, dry cough, shortness of breathe, rapid respiration, nephritis and extreme weakness when their concentration is above the recommended limit [6].However, several methods have been developed to remove heavy metals from industrial waste water before discharge into the water bodies. These methods include reduction, precipitation, ion exchange, reverse osmosis, dialysis and adsorption by coated carbon [7]. Activated carbon was also used and found to be an effective adsorbent though it suffers from the disadvantage of possessing high cost.Several adsorbents like discarded automotive tyres, human hair, starch, xanthate, etc has been successfully tried but were not easily and widely available [1] they also have limited application as they cannot remove metal ions at low concentration within the range of 1-100mg/L [7]. Biosorption of heavy metals from aqueous solution is a relatively new technology for the treatment of industrial waste water [8]. The main advantage of using biosorption technology is due to its cost effectiveness, affordability, availability and environmental friendliness [9]. These materials could be an alternative for expensive waste water treatment process [9]. Very recently, [10] utilized flamboyant tree pod to sorb Hg (II) ion from water and found out that adsorption process follows the pseudo-second kinetic model and the equilibrium data fitted well to both Freundlich and Redlich-Peterson isotherm model.More so, dynamic biosorption of Zn (II) and Cu (II) ions using pre-treated red rose (Rosa gruss) was investigated by [11]. The studies of the removal of copper ions from aqueous solution using dried sun flower as an adsorbent was carried out by [12]. [13] narrated the potential of Nile Rose Plant as an adsorbent to remove metal ions like (Cu2+, Zn2+, Cd2+ and Pb2+) from wastewater within various experimental conditions. Equally,[14] reported that Okra wastes from food canning processes could serve as a potential adsorption of lead removal from various aqueous solutions.Delonix regia (Flamboyant) tree plantation is grown around the world for ornamental reasons, preserving the soil and also conserving the environment. Apart from these benefits, the tree produces flowers in large quantities and at times litters the environment. Developing nations like Nigeria faces solid waste disposal problem, this necessitate the need for the conversion of this waste to useful, value-added products for the removal of metal ions from aqueous solution which would be of benefit to the environment as well as the scientific community in the search for cheaper adsorbent materials.The objectives of this study is to determine the adsorptive capability of Delonix regia flower and establish the adsorption kinetics using pseudo-first and pseudo-second order models for the removal of lead, cobalt and copper ions from aqueous solution.
2. Materials and Methods
2.1. Sample Collection
- The Delonix regia (flamboyant) flowers were collected from different point at the Federal University of Technology, Minna, Bosso campus and also at Bahago secondary school in Minna, Niger state in Nigeria. A composite sample was made from where representative samples were taken for sorption studies.
2.2. Sample Pre-Treatment
- The waste flowers were thoroughly washed with de-ionised water to remove debris. The fresh flowers were sun dried and were grounded using mortar and pestle, it was washed several times with de-ionised water to remove the colour in the substrates. Furthermore, the washed sample was once again dried, ground to fine powder by mortar and pestle then it was then sieved with a mesh size of 300µm to obtain a very fine powder and was kept in an airtight container for further sorption studies.
2.3. Preparation of Aqueous Solution
- A multimetal aqueous stock solution of Pb(II), Cu(II) and Co(II) ions were prepared with their respective salts by carefully weighing out 1.6g of Pb (NO3)2, 3.8g of CuCl2, and 13.04g of CoCl2 and was dissolved together in a 1000 cm3 volumetric flask and diluted with de-ionised water to the mark, which gave a concentration of 1000 mg/L. Successive dilutions was made, first, by preparing concentration of 100 mg/L from the 1000 mg/L stock solution, by pipetting 10cm3 of the solution into a 100 cm3 volumetric flask and making up to the mark with de-ionised water. Successive dilution of the 100ppm was used to prepare other concentrations of 5, 10, 15, 20, 25 and 30 mg/L respectively.
2.4. Multimetal Batch Sorption Experiments
2.4.1. Effect of Contact Time
- 30cm3 of the prepared aqueous solution was measured into a conical flask and mixed with 0.5g of the substrate, well corked and the mixture was constantly shaken in a rotary shaker at 420rpm, with time intervals of 20, 40, 60, 80, 100 and 120 minutes. After each contact time, the mixture was filtered using Whatman filter paper (No. 42) and the concentration of each was determine using Flame Atomic Absorption spectrometer (model, PerkinElmer; Analyst 200).
2.4.2. Effect of pH
- 30 cm3 of the prepared aqueous solution was measured using a measuring cylinder, followed by the addition of 0.5 g of the sample in a conical flask. The pH was varied from 1 to 10. 0.1 M HCl or 0.1 M NaOH was used to adjust the pH of the metal ion solutions to the desired value of interest. The metal solutions containing the biosorbent in the conical flask were well corked and shake using Gallenkamp flask shaker for 60minutes and thereafter the mixture was filtered, and then the concentration of each metal ions removed was determined using Flame Atomic Absorption Spectrometer, (model, PerkinElmer; Analyst 200).
2.4.3. Effect of Initial Metal Ions Concentration
- 30 cm3 of (10, 15, 20, 25 and 30 mg/L) aqueous solution was measured from the prepared 100 mg/L and was mixed with 0.5g of the sample, corked and the mixture was constantly shaken in a shaker at 420rpm for 60 minutes. After constant time intervals, the mixture was filtered using Whatman filter paper (No. 42) and the concentration of each metal ion was determined using Flame Atomic Absorption spectrometer.
2.4.4. Effect of Dosage
- 30 cm3 of the prepared aqueous solution was measured into different conical flasks, followed by the addition of varying masses of the sample (0.5, 1.0, 1.5, 2.0, and 2.5 g) and was well corked. Thereafter it was shaken using Gallenkamp flask shaker for 60minutes, filtered, and then the concentration of each metal ions bound was determined once again using Flame Atomic Absorption Spectrometer.
2.4.5. Calculation of Metal Uptake
- This was used to determine the amount of metal absorbed by the biosorbent.
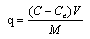 Where, q is the amount of metal ion adsorbed in mg/g; C is the initial metal ion concentration in mg/L; Ce is the final concentration in mg/L; V is the volume of metal ion solution in liters; M is the mass of Delonix regia flower used in gram.The percentage Removal of the metal ion was also determined using;X% =C-Ce/C x100Where, X% is the percentage of metal removed.Kinetics study analysisPseudo-First-Order Kinetics.The rate law is shown below:
Where, q is the amount of metal ion adsorbed in mg/g; C is the initial metal ion concentration in mg/L; Ce is the final concentration in mg/L; V is the volume of metal ion solution in liters; M is the mass of Delonix regia flower used in gram.The percentage Removal of the metal ion was also determined using;X% =C-Ce/C x100Where, X% is the percentage of metal removed.Kinetics study analysisPseudo-First-Order Kinetics.The rate law is shown below: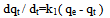 Where, qe and qt are the amount of each of Co(II), Pb(II) and Cu(II) absorbed at equilibrium and time t, respectively. k1 is the rate constant for the pseudo first order biosorption. The integrated law then becomes:
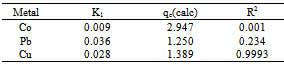
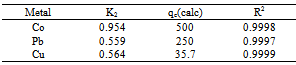
Where, qe and qt are the amount of each of Co(II), Pb(II) and Cu(II) absorbed at equilibrium and time t, respectively. k1 is the rate constant for the pseudo first order biosorption. The integrated law then becomes: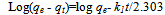 A plot of Log(qe - qt) against time t, was made and values of k1 and qe were obtained from the slope and intercept, respectively.Pseudo-second order KineticsApplicability of the second order kinetics is tested with the rate equation:
A plot of Log(qe - qt) against time t, was made and values of k1 and qe were obtained from the slope and intercept, respectively.Pseudo-second order KineticsApplicability of the second order kinetics is tested with the rate equation: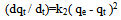 Where k2 is rate law for pseudo second order biosorption.On integrating between the boundary condition of t=0, t=t and q= 0, qe = qt, the following expression was obtained:
Where k2 is rate law for pseudo second order biosorption.On integrating between the boundary condition of t=0, t=t and q= 0, qe = qt, the following expression was obtained: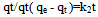 On linearizing,
On linearizing,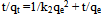 A plot of ( t/qt) against t gives( 1/qe) as slope and (1/k2qe2) as intercept from which k2 can be obtained. Both models tested for suitability using their correlation of coefficient, R2 (Ho and Mckay, 2000, Oyebamiji et al., 2009)
A plot of ( t/qt) against t gives( 1/qe) as slope and (1/k2qe2) as intercept from which k2 can be obtained. Both models tested for suitability using their correlation of coefficient, R2 (Ho and Mckay, 2000, Oyebamiji et al., 2009)3. Results
3.1. Discussion
3.1.2. Influence of pH
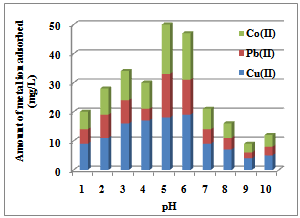 | Figure 1. Effect of pH for the Sorption of Co(II), Pb(II) and Cu(II) ions by Delonix regia |
3.1.3. Effect of contact time
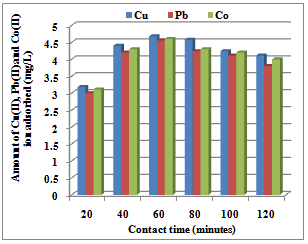 | Figure 2. Contact time for the sorption Co(II), Pb(II) and Cu(II) ions by Delonix regia flowers |
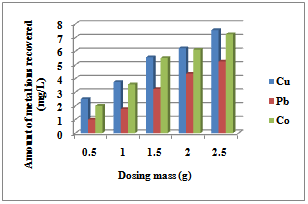
3.1.4. Effect of Adsorbent Dosage
 | Figure 3. Initial Metal ion concentration for the sorption of Co (II), Pb(II) and Cu(II) ions by Delonix regia |
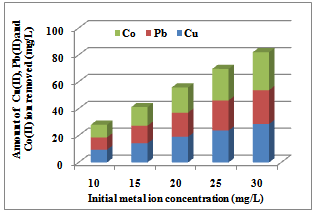
3.1.5. Effect of Initial Metal Ions Concentration
 | Figure 4. Effect of dosage on sorption of Co(II), Pb(II), and Cu(II) ions by Delonix regia flower |
3.1.6. Adsorption Kinetics
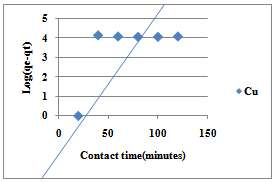 | Figure 5. Pseudo-first order for sorption of Cu(II) ions by Delonix regia |
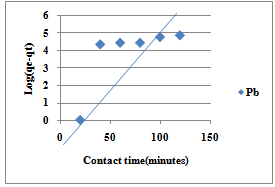 | Figure 6. Pseudo-first order for sorption of Pb(II) ions by Delonix regia |
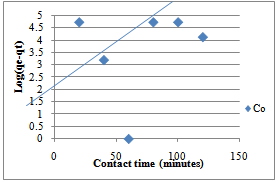 | Figure 7. Pseudo-first order for sorption of Co(II) ions by Delonix regia |
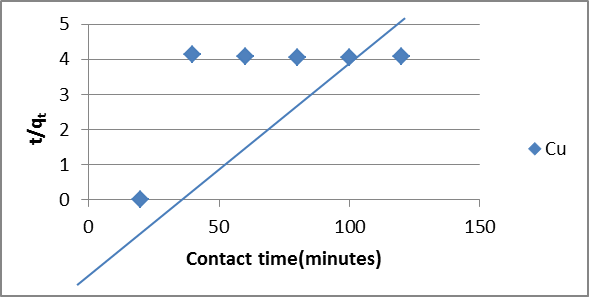 | Figure 8. Pseudo-Second Order for Sorption of Cu(II) ions by Delonix regia. |
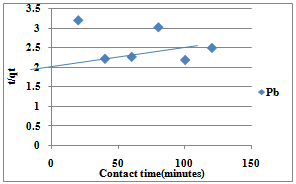 | Figure 9. Pseudo-Second Order for Sorption of Pb(II) ions by Delonix regia |
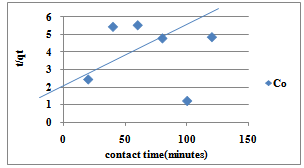 | Figure 10. Pseudo-Second Order for sorption of Co(II) ions by Delonix regia |
|
|
4. Conclusions
- The following conclusion can be drawn from this study,1. The biosorption process was pH, Contact time, dosage and metal ion concentration dependent.2. Contact time of adsorption was found to occur at 60 minutes for all three metal ions.3. Pseudo-First and Second order kinetic model can be used to describe the binding of Cu ions to Delonix regia flower with R2=0.416. However, pseudo-first order can be used to describe the kinetics for the biosorption of Pb and Co ions. That Flamboyant flower could be used an alternative to highly expensive activated carbon to remove the selected metal ions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML