-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(3): 150-156
doi: 10.5923/j.chemistry.20120203.09
Synyhesis and Antibacterial Activities of New 3-Amino-2-Methyl-Quinazolin-4 (3h)-One Derivatives
Abdul Jabar Kh. Atia, Suhair S. Al-Mufrgeiy
Department of Chemistry, College of Science, University of Al-Mustansiriayah, Iraq
Correspondence to: Abdul Jabar Kh. Atia, Department of Chemistry, College of Science, University of Al-Mustansiriayah, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
New derivatives of Quinazolin-4 (3H)-one ring comprising Schiff's bases, 1,3,4-Thiadiazole, 1,3,4-Oxadiazole and 1,2,4-Triazole,Thiaurease moieties are reported. Compounds 3-aminoquinazolin-4(3H)-one (2) was synthesized by reaction of compounds (1) with hydrazine hydrate. Quinazolin-4(3H)-one ester (6) was converted into quinazolin-4(3H)-one hydrazide ester (7) and quinazolin-4(3H)-one thiosemicarbazide ester (8), then compounds (2), (7) and (8) were converted into a variety of derivatives. The structure of these compounds has been established on the basis of their elemental, analytical, and spectral data. These compounds were tested for invitro antibacterial activity against Escherichia coli, Staphylococcus aurousandProteus mirabbilisby standard methods. These synthesized compounds have been shown moderate to good antibacterial activity.
Keywords: Quinazolin-4(3H)-One, 1,3,4-Thiadiazole, 1,3,4-Oxadiazole, 1,2,4-Triazole,Thiaureas
1. Introduction
- The heterocyclic nitrogen compounds like quinazolinone derivatives has a vital role in synthetic drugs and biological processes. A Quinazolin-4-one derivative possessing broad spectrum of biological and pharmacological activities such as antifungal[1], antimicrobial[2], bronchodilator[3] ,antihistaminic[4], anti-inflammatory[5], A series of mannich bases derived from4-[2-methyl-4-oxo-4H-quinazolin-3-yl]benzoic acid show antibacterial, antifungal andanti-inflammatory activities[1].Some Sulpha Drug Quinazolin -4-one Derivatives showed exhibited anti-inflammatory and analgesic activities[6].angiotensin receptor antagonist[7], antiherpes[8],nantitubercular[9], anti- insecticidal[10] and cardiovascular agent[11]. 4- Aminoquinazoline represent as a new class of drugs, it was found good inhibitor to the epidermal growth factor receptor (EGFR)[12,13]. Further more, quinazolines exert their antitumor activity, so used as inhibitor to DNA repair enzyme system, enzyme – mediated repair of strand lesion in DNA is an established mechanism for resistance toward antitumor DNA damaging drugs and radiotherapy[14-16]. So, amino quinazoline can be considered as useful tools to prepare many compounds, the amino group is ready made nucleophilic center for synthesis of condensed heterocyclic ring[17-21]. In the present work we report on the synthesis of compounds derived from 3 – amino quinazoline comprising various moieties on amino group such as1,3,4-Thiadiazole,1,3,4-Oxadiazole and 1,2,4-Triazole,Thiaurease with the purpose of furtherinvest igation of their possible antibacterial activities.The structures of all compounds have been evaluated by elemental analysis and spectral analysis (IR and U.V)
2. Results and Discussion
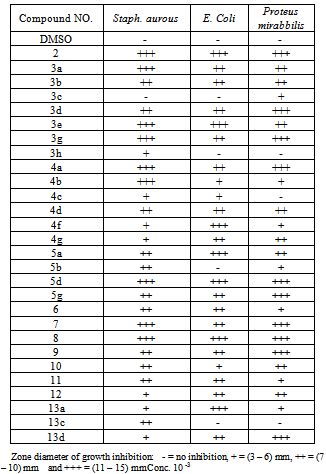
- New 3-amino quinazoline – 4 – one derivatives were prepared following the reaction sequences depicted inschemes 1.In the first part of this study2-methyl-4-(3H)-benzoxazin-4-one was obtained from the reaction of anthranilic acid with acetic anhydride, the reaction proceeds by the attack of the nitrogen of amine at the carbon of carbonyl of acetic anhydride and forming the ammonium cation, however, ammonium cation is unstable, so the H+ transfer to oxygen inion to give (-OH) group. Then the (H2O) will be lose to give azomethene moiety and afforded the stable compound. The IR spectrum showed the C=O stretching absorption at 1710 cm-1 and two absorption band at 2825-2980 cm-1 for the C-H alph. U.V spectrum showed two intense absorption maxima at 313 nm and 256 nm which tenderly attributed to n → π* and π → π* electronic transitions respectively. The elemental analysis showed the theoretical values nearly found values. Reaction between corresponded compound (1) and hydrazine hydrate afforded the compound (2) in good yield. The structure of (2) was confirmed by the presence of (NH2) stretching vibration at 3200-3450 cm-1 , in addition to the band at 1695 cm-1 for the (C=O) absorption band. UV spectrum exhibited two distinguishable maxima near 309 nm and 243 nm which clearly due to n → π* and π → π* electronic transitions respectively. The theoretical values of elemental analysis which were closely to the found values. Condensation of the amino group of derivative (2) with appropriate aldehydes or ketones in absolute ethanol gave the Schiff's bases (3a-h). The formation of these Schiff's bases was indicated by the presence in their IR spectra of the isomethine (CH = N) stretching band at 1595-1655 cm-1 combined with the disappearance of NH2 stretching bands. The UV. Spectra of the Schiff 's bases mostly showed two intense maxima at 291-359 nm and 233-286 nm which belong to n → π* and π → π* electronic transitions respectively. The elemental analysis of someSchiff's bases showed the close values to the theoretical values. The reaction of Schiff's bases (3a-f, 3h) with 3,5-dinitrobenzoyl chloride in dry benzene afforded the derivatives (4a-g). The appearance of IR new absorption bands in general in regions 1350-1580 cm-1 and 750-800 cm-1 were tentatively belong to (NO2) and (C-Cl) respectively. UV.Spectra of compounds (4a-g) showed intense bands at 255-322 nm and 202-239 nm which belong to n → π* and π → π* electronic transitions respectively. Moreover, heating compounds (4a-g) under reflux with thiourea in the presence of Na2CO3 for (5 hrs.) lead to the nucleophilic substitution of (Cl) by (Na+ S- ) of thiourea and compounds (5a-g) were formed .These compounds (5a-g) were characterized by their IR , UV.Spectra and elemental analysis for some compounds. New doublet absorption bands in the region 3100-3440 cm-1 attributed to (NH2 and NH) functional moieties, other characteristic bands in the region 720-790 cm-1 correlated to C-S moiety. Moreover the band of C-Cl around 750-800 cm-1 has disappeared. U.V. Spectra of compounds (5a-g) showed intense bands at 228-331 nm and 202-218 nm which belong to n → π* and π → π* electronic transitions respectively. Condensation of compound (2) with ethyl bromoacetate to give the corresponding compound(6). The reaction is followed by disappearance of doublet absorption bands of (NH2) at 3200-3450 cm-1 and appearance two sharp absorption bands, one of which appeared at 1730 cm-1 was attributed to carbonyl function of ester and the other observed at 1700 cm-1 was assigned to (C=O) stretching frequency corresponding to the lactam ring carbonyl. The spectral data of UV of compound (6) showed two maxima at 348 nm and 235 nm due to n→π* and π →π* electronic transition respectively. The treatment of compound (6) with hydrazine hydrate did not produce expected compound (I) instead compound (7) formed as reaction product. In the initial stage of this reaction hydrazine hydrate attack ester carbonyl and followed by the elimination of molecule C2H5OH and as result compound (7) are obtained. The structure of compound (7) was confirmed by the presence of (NH, NH2) stretching vibration at 3120cm-1 and 3180-3315 cm-1 respectively, and decreasing value of vibration of amide carbonyl to 1610 cm-1 .UV. spectrum of compound (7) showed two intense maxima at 346 nm and 261 nm which belong to n → π* and π → π* electronic transitions respectively. Acid hydrazide can be considered as useful intermediates leading to the formation of some heterocyclic rings such as 1,2,4-triazol (12) and 1,3,4-oxadiazole (10) were synthesized from the reaction of compound (7) with carbon disulfide in the presence of potassium hydroxide (scheme 2) .The IR spectrum of (10) showed disappearance of carbonyl group of acid hydrazide at 1610 cm-1 and appearance of band at 2580 cm-1 due to (SH) moiety and band at 1273 cm-1 for (C=S) . UV.spectrum of compound (10) showed intense bands at 336 nm and 250 nm which belong to n → π* and π → π* electronic transitions respectively. The spectral data of IR of compound (12) showed two bands at 3140-3275 cm-1 was attributed to (NH2) in additional to the band at 2600 cm-1 for (SH) absorption band. UV.spectrum exhibited twodistinguishable maxima near 336 nm and 245 nm which clearly due to n → π* and π → π* electronic transitions respectively. Reaction between corresponded compound (6) and thiosemicarbazides givesthiosemicarbazone derivative (8) in good yield. The structure of compound (8) was confirmed by the appearance of (NH, NH2) stretching frequency at 3170 cm-1 and 3260-3335 cm-1 respectively and reduce the vibration of ester carbonyl to 1650 cm-1. UV.spectrum showed intense absorption maximum at 348 nm and 232 nm which tenderly attributed to n → π* and π → π* electronic transitions respectively. New 1,3,4-thiadiazole derivative (9) containing amino moiety was prepared from the reaction of compound (8) with H2SO4. The IR spectrum showed two bands at 3100-3380 cm-1 due to (NH2) and band at 617nm which clearly due to (C-S-C). finally compound (7) was also allowed to react with three different aldehydes in absolute ethanol to give corresponding Schiff 's bases (13a-d). The reaction is supported by the disappearance of (NH2) band at 3315-3180 cm-1 and the appearance of isomethine (CH=N) at 1595-1630 cm-1 .The UV. spectra showed two maxima at 257-303 nm and 203-216 nm due to n → π* and π → π* electronic transitions respectively.Structure - inhibition relationshipThe antibacterial activity of the quinazolin-4-(3H)-one derivatives was tested by agar disc-diffusion method against Staph. Aurous, E. Coli and Proteus mirabilis bacteria. Dimethylsulphoxide (DMSO) was used as solvent control, the concentration of tested compounds (10-3). Table 1summarize the inhibition results of the study compounds. The results indicated that some compounds had hardly inhibition and the inhibition was increased or decreased with changing the substituent groups of compounds. It could be observed that all the tested Compounds were active toward proteus. Mirabilis, except compounds 3c and 4c.So we can to say, the decrease activity of inhibition of compounds 3c and 4c because these compounds have nitro group which it electron withdrawing while Change of the nitro group with OH group make compound has high inhibition, that is to say, inhibition activity was increased with donate group and was decreased when the same group had electron withdrawing. All the tested compounds were active toward E. Coli. except compounds 3c, 3h, 5b and 13c. And all the tested compounds were active toward Staph. Aurous except compounds 3h,4c and 13c. On the other hand compounds 2, 5d, 8 shohigh inhibition toward kinds of bacteria tested. In additioncompounds 3a, 3e, 3g, 4a, 4b, 5d, 7, 8, compounds 3e, 4f, 5a, 5d, 8, 13a, compounds 3d, 3g, 4a, 5d, 5g,7, 8, 9 showed high inhibition toward Staph. Aurous, E. Coli and Proteus mirabbilis
|
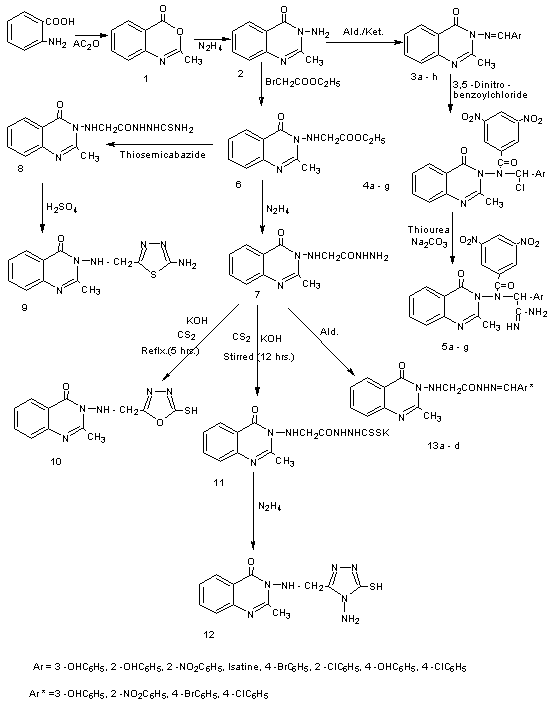 | Scheme 1. The synthesis of compounds 1 – 13a–d |
3. Experimental
- All chemicals are of analar grade (Merk , Fluka) and used without further purification. Melting point were determined in open capillary tubes on a Galten Kamp melting point apparatus and are uncorrected. FT-IR spectra (KBr discs) were recorded with 8300 Shimadzu in the range (4000 – 600) cm-1. The (C.H.N) elemental analysis were done using (C.H.N) Carlo Erba 1106 elemental analyzer. UV. Spectra were recorded on Hitachi 2000 spectrophotometer using absolute methanol as solvent.Synthesis of 2-methyl – 4H- 1,3 benzoxazin – 4 – one (1)[22] A mixture of anthranilic acid (0.01 mole, 2 gm) and acetic anhydride (10 ml) was refluxed for 1 hr., then the mixture was cooled to room temperature. The product was collected and recrystallized from water. Yield% 94, m.p(298-300), Synthesis of 3-amino-2-methylquinazolin-4-(3H)-one (2)[22]To a solution of compound (1) (0.02 mole, 3 gm) in (15 mL) absolute ethanol, hydrazine hydrate (99%) (10 ml) was added. The mixture was refluxed for (27 hrs.), then the mixture was cooled and the precipitate was filtered and recrystallized from water. Yield% 79, m.p(220-222).Synthesis -3-[ (arylidene) amino ] -2-methylquinazolin -4- (3H) -one (3a-h)
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML