-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(3): 131-136
doi: 10.5923/j.chemistry.20120203.06
Dynamics of Apolar Guest Solubilized in Bile Salt Micelles: Photochemistry of Acenaphthylene as a Probe to Understand the Supramolecular Characteristics of the Aggregates
Nick Carter, Mahesh Pattabiraman, Luis Albert Arias
Department of Natural Sciences, Western New Mexico University, Silver City, 88061, USA
Correspondence to: Mahesh Pattabiraman, Department of Natural Sciences, Western New Mexico University, Silver City, 88061, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Product distribution resulting from the excited state reactivity of acenaphthylene is influenced predominantly by multiplicity of the excited state, mobility of the substrate in a given medium, and spatial constraints. The photochemistry of acenaphthylene solubilized by sodium cholate, a member of the atypical micelle forming molecules called bile salts, in water was investigated to understand the interior of the aggregates. Results indicate that the supramolecular characteristics of the aggregates change significantly with concentration which in turn affects the mobility of the encapsulated acenaphthylene guest leading to changes in product distribution from the reaction.
Keywords: Bile Salts, Sodium Cholate, Micelles, Complexation, Photochemistry, Acenaphthylene, Excited State, Supramolecular Chemistry, Green Chemistry
Article Outline
1. Introduction
- Micelles are supramolecular systems with tremendous applications in biotechnology, catalysis, medicine, nanote- chnology, food science, environmental remediation, catalysis, and other areas of practical significance.1-8 Straight chain amphiphiles such as phospholipids, alkyl sulfonates, tetra alkyl ammonium salts, and alkyl polyglucosides have been well studied for their micelles. But bile salt micelles have remained relatively unexplored,9,10 especially for application in green chemistry as media to perform chemical transformations.11-13Bile salts are produced in the liver of mammals and are understood to play important roles in digestion of fat.14-17 Bile salts are family of compounds with steroidal frame work and contain hydroxyl groups (two to three) and an acid group containing side-chain. The steroidal backbone of the molecule assumes a puckered shape in which the hydrophobic methyl groups occupy the convex side and the hydrophilic hydroxyl groups occupy the concave side (Figure 1) rendering the molecules amphiphilic. When dissolved in aqueous solutions at concentrations close to their formal CMC, the bile salts aggregate (Figure 2) to produce primary aggregates composed of 5-10 monomers18 in which theconvex side faces the interior of the aggregate and the concave side faces the aqueous phase. Furthermore, at higher concentrations the primary aggregates are known to aggregate further to yield larger loosely held structure called the secondary aggregates sustained by hydrogen-bonding between the hydroxyl groups of the bile salt and water molecules in the medium.18-20
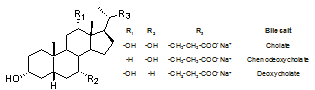 | Figure 1. Chemical structure of bile salt monomers |
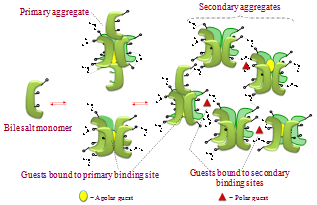 | Figure 2. Representation of stepwise aggregation of bile salts in aqueous medium resulting in formation of primary and secondary aggregates |
2. Results and Discussion
2.1. Photochemistry of Acenaphthylene
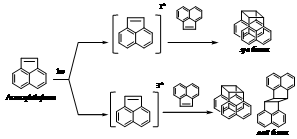 | Scheme 1. Photochemistry of acenaphthylene |
2.2. Inclusion of Acenaphthylene within Sodium Cholate Aggregates
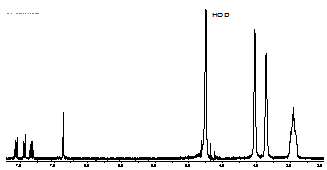 | Figure 3. Partial 1H NMR spectrum of acenaphthylene (0.66 mM) solubilized in D2O by sodium cholate aggregates (45 mM) shows the aggregate mediated dissolution of guest in aqueous medium |
2.3. Photoreactivity of Acenaphthylene in Sodium Cholate
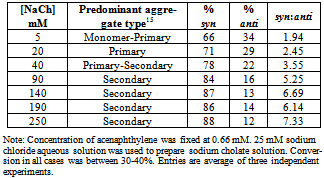
- Table 1 presents the product distribution resulting from the irradiation of solutions in which concentration of NaCh was varied incrementally (5 mM to 250 mM) while that of the guest ACN was maintained at 0.66 mM. The concentration range for NaCh in the experiments were chosen to entail equilibrium situations in which the predominant equilibrium species shift from NaCh monomer (at lower concentrations) to primary aggregates to secondary aggregate (at higher concentrations).
|
|
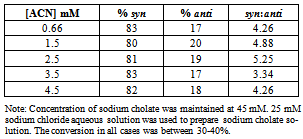
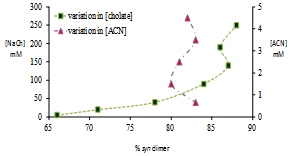 | Figure 4. Photoproduct distribution from reaction of ACN and variation with concentration changes |
|
2.4. Discussion of Results
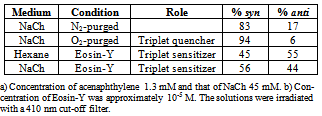
- Selectivity in product distribution of acenaphthylene in constrained medium is shown to be primarily controlled by two factors: the spin states of ACN in the excited state,21,22 and the availability of space, where spatial limitations favor the more compact syn dimer over anti.28 The possibility of a ground state complex of acenaphthylene preoriented to form the syn dimer could be ruled out based on the reports by Bohne et. al.27 as this would require multiple occupancy for ACN within the primary aggregates of sodium cholate – primary binding sites are too small to accommodate two guest molecules simultaneously.To gauge the influence of the spatial factor alone on the product distribution, the contribution from the spin factor was eliminated by using triplet quenchers and sensitisers (Table 3). In the absence of spin state manipulating species, ACN reacts to yield 83% syn and 17% anti dimer. Whereas, the same for oxygen purged solution (oxygen being a triplet quencher) yielded almost exclusively syn dimer. The formation of as much as 94% of syn dimer in the medium is indicative of the fact that the medium does not thwart the near quantitative formation of syn dimer.On the other hand, when the reaction was performed with Eosin-Y in the medium, a triplet sensitizer, yielded 56 % syn dimer; comparing this with the Eosin-Y sensitized reaction in hexane where no spatial constraints exist, 11 % more syn dimer (Table 3) was obtained within the aggregates. Considering the results from control studies presented in table 3, it is evident that the formation of syn dimer is favored within the primary aggregates most likely due to its compactness in shape, as the space available for binding within the aggregates is both limited and very rigid.The notable feature in the photodimerization of acenaphthylene within in sodium cholate micelles is the observed trend of increase in syn dimer formation within increase in micelle concentration until 90 mM and no significant changes thereafter (Figure 4). By correlating the bile salt equilibrium dynamics with the trend in syn:anti proportion in reaction outcome with changes in NaCh concentration, it is apparent that as the concentration of bile salt increases, the equilibrium shifts towards the secondary aggregates formation the photochemistry of acenaphthylene is steered towards the formation of syn dimer in higher proportion. In order to explain the observed trend in product distribution, we propose the following: acenaphthylene, being highly nonpolar, is expected to be solubilised in primary aggregates only. At low concentrations of NaCh secondary aggregates is expected to be absent and, therefore, ACN will be solubilised mostly by primary aggregates. In this situation, the probability of dimerization of ACN in its S1 and T1 states are limited by the diffusion of the primary aggregates – the mobility of the guest is only as fast as the mobility of the primary aggregate itself. Since the S1 state is short-lived than the T1 state (by three orders of magnitude) the low NaCh concentration situation favors reactivity from the T1 state more than the S1 as the latter would be the first to “die out” due to relaxation.
3. Conclusions
- Sodium cholate aggregates were employed to perform the photochemical dimerization of acenaphthylene.Concentration variation experiments and control experiments were performed to understand the interior of the primary aggregates and the shift in supramolecular characteristics of the bile salt micro-heterogeneous medium. Results indicate that the tight spaces within primary aggregates favor the formation of syn dimer over the spatially demanding anti dimer. In addition the concentration dependent photochemistry suggests that at lower concentrations of NaCh the mobility of the guest is limited by the diffusion characteristics of the primary aggregates. Whereas, at higher concentration of NaCh the predominance of secondary aggregates act as a micro-network of primary aggregates increasing the mobility of the guest molecules. The reaction provides insight into the mobility and spatial constraint of apolar guest molecules encapsulated within the bile salt micellar system.
4. Experimental
- MaterialsSodium cholate (NaCh), sodium chloride, anhydrous sodium sulfate, and eosin-Y, were procured from Sigma Aldrich Co. and used without further purification. Acenaphthylene procured from Sigma Aldrich Co. was 95% acenaphthylene and 5% acenaphthene as indicated in the manufacturer’s label and analyzed by us in the GC. The compound was purified by vacuum sublimation technique during which acenaphthylene sublimed on the upper walls of the sublimator while acenaphthene remains at the bottom. This procedure was repeated to obtain acenaphthylene more than 98% pure.Mass Balance Experiments:Mass balance experiments were carried out to confirm that product selectivities observed were not due to selective decomposition, loss, inclusion, or exclusion of reaction components. A standard solution with equal amounts of the acenaphthylene and an external standard (2mg each in 5mL of hexane-ethyl acetate) for GC (naphthalene) was prepared and injected in the GC. The ratio of the peak intensities was taken as a standard for 1:1 mixture by mass. To determine the mass balance after photochemistry, the irradiated samples were extracted and mixed with an equal mass (as that of the reactive guest taken) of internal standard and analyzed in the GC. The ratio of all peaks in the GC run with respect to the internal standard was considered to calculate the mass balance. In all cases the mass balance was estimated to be at least 80%.Complexation, irradiation and extraction procedure:A standard solution of acenaphthylene (5mg/mL) in dichloromethane was prepared. Volume corresponding to 0.5 mg was pipetted out and solvent evaporated to obtain a thin film of the compound. 20 mL of 25 mM sodium chloride solution, 45 mg of sodium cholate were added to the acenaphthylene thin film. Sonication for 15 minutes and allowing the solution to stir for 2 hours resulted in formation of clear yellow colored solutions. The solutions were filtered with a Whatmann filter paper of fine porosity and slow flow rate. The filtrate was purged with nitrogen for 15 minutes, sealed and irradiated with a 410 nm cut-off filter. The cut-off filter was used to prevent dimers from absorbing any radiation from the photochemical lamp source.After irradiation, the solution was diluted by adding 30 mL of deionized water. Extraction of the starting material and reactants was performed in a separatory funnel with 30 mL of ethyl acetate and 15 mL of acetonitrile mixture. The organic layer was dried over anhydrous sodium sulfate, and analyzed in a Shimadzu model 4020 GC-MS fitted with an Agilent SE-30 column. The identities of the dimers were also established by 1H NMR spectroscopy.Reported valuesAll values reported in tables are averages of three independently performed experiments. The averages are rounded off to the closest unit and the error limit for reported values is ± 3%.Analysis of photodimers of acenaphthylene:The identities of the photoproducts were confirmed by NMR spectroscopy recorded on a Bruker Avance 300 MHz spectrometer and referenced using the residual solvent signal as the internal standard. The chemical shifts were matched with those reported in literature.22Syn dimer: 1H NMR (CDCl3) δ 7.18 (d, 4H, J = 8.0 Hz), 7.15 (d, 4H, J = 7.5 Hz), 7.03 (d, 4H, J = 7.3 Hz), 4.85 (s, 4H).Anti dimer: 1H NMR (CDCl3) δ 7.74 (d, 4H, J=8 Hz), 7.60 (d, 4H, J = 7.9 Hz), 7.01 (d, 4H, J = 7.9 Hz), 4.11 (s, 4H).For routine analysis and quantitative determination of products and reactant composition in the reaction mixture, samples were analyzed as such after extraction in a GC-MS. GC-MS program: Initial temp 100˚ C, Initial time 1 minute, Initial rate 10˚ C/minute, final temperature 300˚ C and final time 5 minutes. Retention times: ACN at 7.5 min, syn dimer at 13.3 min and anti dimer at 16.1 min.
ACKNOWLEDGEMENTS
- The work presented in the manuscript was performed with the financial support from VP’s office at WNMU. MP thanks Mr. Ross Fischer in the Dept. Of Natural Sciences, WNMU for his support, and Dr. Gopalan (New Mexico State University, Las Cruces) for helping us in acquiring NMR spectra for characterization. MP is very thankful to Dr. V. Ramamurthy for providing insight and advice in this project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML