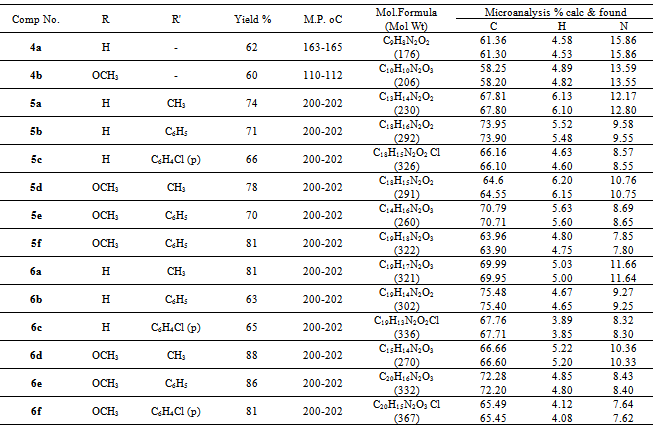
-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(3): 115-120
doi: 10.5923/j.chemistry.20120203.03
Microwave-Assisted Synthesis and Pharmacological Activity of Pyrazolyl Benzofuran Deravatives
Raghunandan Deshpande1, Bhagawan Raju M2, Parameshwar S3, Shanth Kumar S.M4, Appalaraju S1, Manjunath S Yelagatti5
1H.K.E.S’s Matoshree Taradevi Rampure Institute of Pharmaceutical Sciences, Sedam Road, Gulbarga-585105, Karnataka, India
2CM College of Pharmacy, Maisammaguda, Dulapally, Hyderabad-500014, Andhra Pradesh, India
3Department of Chemistry, Gulbarga University, Gulbarga 585106, Karnataka, India
4V.L. Colleges of Pharmacy, Manik Prabhu Temple Road, Raichur-584102, Karnataka, India
5Sri Krupa institute of Pharmaceutical Sciences, Village Velkatta, Siddipet-502277, Medak, Andhra Pradesh, India
Correspondence to: Raghunandan Deshpande, H.K.E.S’s Matoshree Taradevi Rampure Institute of Pharmaceutical Sciences, Sedam Road, Gulbarga-585105, Karnataka, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Microwave-assisted Organic Reaction Enhancement (MORE) has emerged as a new ‘lead’ in organic synthesis. During the studies we observed that microwave –assisted organic synthesis requires 2-4 min. of time whereas the conventional ones takes 4-6 hrs and often with poor yields. Hence, with an objective of reducing the reaction time, application of microwave technique has proved to be advantageous in case of synthesis of new benzofuran derivatives. The structures of synthesized derivatives emerged is confirmed by their IR, NMR and Mass spectra. The representative compounds synthesized with this route is further screened for anti-bacterial, analgesic, anti-inflammatory and ulcerogenic studies. In this study we have observed that the benzofuran derivatives are less ulcerogenic compared to phenyl butazone. The structural comparisons have revealed that the chloro substitution will increase the ulcerogenic toxicity.
Keywords: Analgesic, Anti-Inflammatory, Antimicrobial, Microwave-Assisted Synthesis, Pyrazolyl Benzofuran, Ulcerogenic Toxicity
Article Outline
1. Introduction
- In the recent years, microwave-assisted organic reactions have emerged as a new ‘lead’ in modern organic synthesis technology[1-5]. Important advantage of this technique is highly accelerated rate of the reaction with improved quality and quantity of the product. The technique offers simple, clean, fast, efficient, and economical and environment friendly method for the synthesis of number of organic molecules. Moreover, the technique is considered as an important ‘green chemistry’ approach because of its eco-friendly nature[6-9]. Conventional methods of organic synthesis usually need longer heating time, elaborate and tedious apparatus set up, resulting in higher cost of production. Excessive use of solvents/ reagents leads to environmental pollution Efforts are being made to establish the green chemical transformations and to develop several new effective microwave-induced methods for the synthesis of organic compounds[10-12]. The energy used in microwave irradiation is same as that of thermal energy. The advantage in application of this method is its completion in short dura tion and operational simplicity. The use of single frequency microwave oven for this is well established in MORE(Carroet al) (Microwave Induced Organic Reaction Enhancement) chemistry. The experiments like determination of saponification value, loss on drying could be performed within minutes[13,14].Pyrazolobenzofurans which carried off only synthetic interest, initially, are now finding greater importance as biologically active molecules[15]. Some of these compounds are reported to possess bactericidal, anti-viral and anti-cancer activity[16,17]. Annulation of pyrazole ring system on benzofuran nucleus has resulted in compounds possessing analgesic and anticonvulsant activities[18]. In view of these findings, we focused our interest on benzofuran coupled with pyrazole for our research program. The carbonyl hydrazide is used for the development of pyrazole ring system (as quoted in literature for having best functionality). The synthetic strategy that involves the modification of carbonyl hydrazide group located on the furan moiety of benzofuran nucleus in to the desired pyrazole ring system. 3- methoxy-2-carbohydrazide has chosen for this investigation. The reaction on condensation of benzofuran-2-carbonylhydrazide with different acetones and chloroacetones and further treated with DMF and POCl3 under microwave irradiation.Benzofuran-2-carbonyl hydrazine and pyrazoles are biologically active, synthetically useful and important heterocyclic compounds[19,20]. In view of this, we report here the microwave assisted synthesis of pyrazolyl benzofurans and screened for different pharmacological activities.
2. Materials and Methods
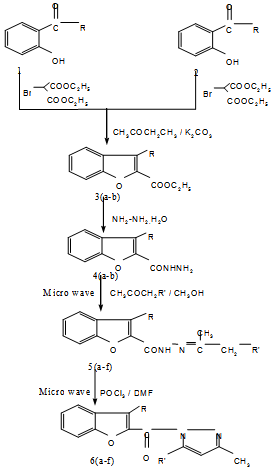 | Scheme 1. Microwave mediated synthesis of different pyrazolyl benzofuran derivatives from Benzofuran-2-carbohydrazide |
|
 The procedure is followed as per given in[24]. Albino rats (175-185 g) were divided into different groups consisting of six animals in each group. Ulcerogenic activity evaluated after the administration of test compounds or Phenyl butazone at the dose of 50 mg/kg. Control rats received administration of vehicle (suspension of 2% acacia). Food but not water was removed 24 h before administration of the test compounds. After the drug treatment, the rats were fed normal diet for 17 h and then sacrificed. The stomach was removed and opened along the greater curvature, washed with distilled water and opened along the greater curvature, washed with distilled water and cleaned gently by dipping in saline. The gastric mucosa of the rats was examined using 10X microscope. The lesions were checked, counted and categorized into perforated (greater than 2mm in diameter), average (1-2 mm) and small (less than 1 mm). For each stomach the severity of mucosal damage was assessed according to the following scoring system. The mean score of each treated group minus the mean score of the control group was considered as the ‘ulcer index’ of gastric damage. The score on the performance of the respective compounds based upon their ulcerative response i.e., more the score less the ulcerogenic response.
The procedure is followed as per given in[24]. Albino rats (175-185 g) were divided into different groups consisting of six animals in each group. Ulcerogenic activity evaluated after the administration of test compounds or Phenyl butazone at the dose of 50 mg/kg. Control rats received administration of vehicle (suspension of 2% acacia). Food but not water was removed 24 h before administration of the test compounds. After the drug treatment, the rats were fed normal diet for 17 h and then sacrificed. The stomach was removed and opened along the greater curvature, washed with distilled water and opened along the greater curvature, washed with distilled water and cleaned gently by dipping in saline. The gastric mucosa of the rats was examined using 10X microscope. The lesions were checked, counted and categorized into perforated (greater than 2mm in diameter), average (1-2 mm) and small (less than 1 mm). For each stomach the severity of mucosal damage was assessed according to the following scoring system. The mean score of each treated group minus the mean score of the control group was considered as the ‘ulcer index’ of gastric damage. The score on the performance of the respective compounds based upon their ulcerative response i.e., more the score less the ulcerogenic response.3. Results and Discussions
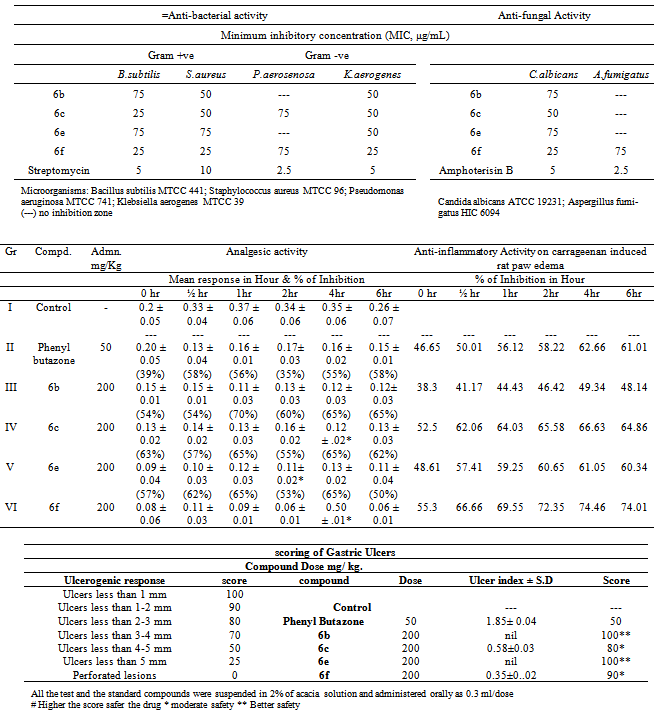
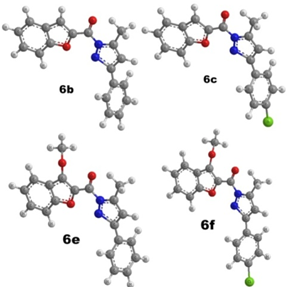
- The derivative obtained from pyrazolylbenzofuran basic nucleus in two step synthesis, their structures was confirmed by IR, 1HNMR and Mass spectra. For brevity only 4 synthesized derivatives which have shown satisfactory in-vitro and in-vivo biological activities were reported.In vitro activityAnti-bacterial & anti-fungal activityIn the series, representative compounds 6b, 6c, 6e and 6f analyzed is shown in table 2. The compound 6f is highly active against all the gram-positive bacteria and gram negative bacteria, the compound 6c were active against B. subtilis. It is interesting to note that the compounds which were substituted with chloro at 4th position of pyrazolyl ring, displayed notable antibacterial activity.In anti-fungal analysis, compound 6f has shown satisfactory activity against Candida albicans and moderate activity against Aspergillus fumigatus. All other compounds (6b, 6cand 6e) did not indicate any positive sign in this experiment.In vivo activityAnti- inflammatory activity & Analgesic activityThe pharmacological screening of the tested compounds showed anti-inflammatory activity ranging from 38.33 to 78.11% (Table 3), whereas the standard drug phenylbutazone showed 79.5% inhibition after 4 h. The anti-inflammatory activity of pyrazolylbenzofuran derivatives synthesized using microwave exposure of compound 6b, 6c, 6e and 6f ranged from 52.5 to 74.5%. The compounds 6c and 6f which are chloro substituted showed higher activity than the standard drug phenylbutazone, whereas in the compound 6b, the anti-inflammatory activity decreased. Also, it was observed that the compound 6e showed activity ranging from 48.1 to 66.63%, which is nearly equivalent to the standard drug phenylbutazone. It is clear from Table that the presence of 3-methoxy group and further chloro substitution at 4th position increases the anti-inflammatory activity.All the four compounds have anti-inflammatory activity than those of the standard were further tested for their analgesic activity at a dose of 50 mg kg–1 phenylbutazone (Table. 1). Compounds showed analgesic activity ranging from 58.4 to 72.7%, whereas the standard drug ibuprofen showed 69.5% inhibition. The results followed the path equal to that of analgesic activity as the chloro substitution even in this case enhanced the analgesic activity, however with a degree of variation.Ulcerogenic activityIt is noted that though the antimicrobial, analgesic and anti-inflammatory activities increased with chloro substitutions on pyrazolyl ring system the ulcerogenicity profile is also slightly increased (Table 4). The toxicity profile is almost negligible on comparison with standard drug phenylbutazone. The compounds 6b and 6e which had shown moderate analgesic and anti-inflammatory activity had shown nil GI perforations.
|
4. Conclusions
- Meaningful, safe and economical synthesis of pyrazolyl benzofuran derivatives are synthesized with microwave-assisted green chemical technology. The high yield and the better pharmacological activity and considerable in-vivo tolaratability encourages for the further research in the same line. In the developed series of derivatives, from the structural activity relationship infers that with the chloro substitution to the pyrazolylbenzofuran derivatives enhances the biological activity but with the slight increase in ulcerogicity profile. However it is negligible when compared to the commercially available standard drug, phenylbutazone.
ACKNOWLEDGEMENTS
- Our gratitude to Late Shri. Ghevarchand Suranaji, Managing Director, Micro Labs. Ltd., for the moral support. We acknowledge IISc, Bangalore for providing spectra and Biogenics, Hubli for providing research facilities. Raghunandan Deshpande thank his father for editing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML