-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(2): 94-98
doi: 10.5923/j.chemistry.20120202.14
Solvents Design on the Basis of Molecular-Microscopic Properties of Binary Mixtures for Lycopene Extraction
Hadi Salari 1, Hanie Rohani 2, Mohammad Reza Elahifard 1, Mohammad Hosseini 2, Mohammad Reza Gholami 1
1Department of Chemistry, Sharif University of Technology, Azadi Ave., Tehran, P.O.Box 11365-9516, Iran
2Department of Chemical Engineering, Amirkabir University of Technology, Tehran, Iran
Correspondence to: Mohammad Reza Gholami , Department of Chemistry, Sharif University of Technology, Azadi Ave., Tehran, P.O.Box 11365-9516, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
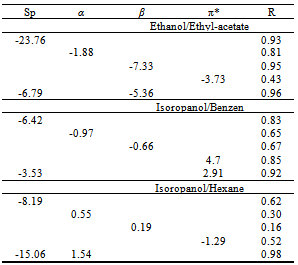
Lycopene is a natural compound with high-value of nutritional and medical properties. Nowadays, the extraction of Lycopene with mixtures of solvents to obtain more efficiency and less toxicity is interested. In this study, the Lycopene extraction in three binary mixtures of solvents (Ethyl-acetate/Ethanol, Hexane/Isopropanol and Benzene/Isopropanol) was measured and then the effects of solvatochromic parameters (π*, dipolarity/polarizability; β, hydrogen-bond acceptor basicity; α, hydrogen-bond donor acidity) and solvophobicity (Sp) parameters on the extraction from tomato tissues have been considered. Among the all parameters, Sp shows the biggest contribution in the extraction that is well justified, knowing that Lycopenes are lypophobic. Our calculation results show that in addition to Sp, another parameter is needed to get the reasonable correlation between the parameters and the extraction. For Ethanol/Ethyle-acetate mixture, β, for Isopropanol/Benzene mixture, π* and for Isopropanol/Hexane mixture, α, participated in the correlation equation in addition to Sp.
Keywords: Lycopene, Extraction, Solvatochromic Parameters, Solvophobicity
Article Outline
1. Introduction
- Carotenoids, the most important natural pigments found in becteria, plants, fungi and animals[1-3] cannot be synthesized by human; hence, they must be obtained from dietary sources. Lycopene as a carotenoid with antioxidant properties is accumulated in photo-synthesis pigment-pigment complex of plants that can be most easily seen in a ripe tomato, water melon, pink grape fruit, guava and papaya, giving them a characteristic red color[1-5]. Tomato and tomato products are the major source for Lycopene production. The molecular structure of Lycopene consists of a long chain of conjugated carbon-carbon (11 conjugated double bonds) having various geometrical isomer; the all trans- isomer are of the dominate percentage in most raw material but the cis-isomers are more compatible in our body and with even stronger bioactivities[6,7]. In-vitro and in-vivo studies showed that Lycopene was promising bioactive components on lowering own the risk of some chronic diseases including certain cancer (e.g. prostate cancer) and coronary heart diseases[6].The supplying of this product relies on the extraction of Lycopene from plants and zymotie liquid via chemical extraction with conventional solvent (that may assist with supersonic or microwave instruments), and super critical fluid (CO2) extraction (SCFE)[4,8]. SCFE represents non- organic solvent residing as an advantage but the equipment and the energy consumption is very high. Due to the hydrophobic nature and limited solubility of carotenoids in water, the selection of appropriate solvents has been recently subjected of large studies[9,10]. There are two important factors to select the best solvent, i.e. 1) extraction efficiency and 2) complete removing and less adverse effects on human health. Unfortunately, most solvents with high extraction efficiencies are considered to be toxic. This represents the mixture of solvents to obtain higher extraction with lower toxicity.Although some solvents mixtures were used in caroteniods extraction[1,2], the investigation of solvent-solvent interactions and their impress on the extraction of various caroteniods have not been considered. Solute–solvent interactions developed in solvation shell of the solutes, cause physical and chemical effects in many processes. These effects of the medium are originated from changes in its polarity, solvophibicity and hydrogen bonding[11-14]. Interactions in mixed solvents are much more complex than in net solvents because a solute can be surrounded preferentially by the mixture components or formed complex [11]. The behaviour of solvents in chemical processes can be understood with an investigation of the solution interactions with a solute. Solvatochromic parameters which studies solute-solvent interactions can show specific and non specific interactions in mixed solvents[11,12]. In this study we applied Ethyl-acetate/Ethanol, Hexane/ Isopropanol and Benzene/Isopropanol mixtures to lycopen extraction. On the basis of proper probes, Solvophibicity (Sp) and three solvatochromic parameters (i.e. π*, dipolarity/polarizability; β, hydrogen-bond acceptor basicity; α, hydrogen-bond donor acidity) are followed in net and mixed solvents versus extraction and then, the best correlation between them has been calculated and discussed.
2. Experimental
- Lycopene with 97% purity was supplied from Sigma Aldrich, USA. All solvents, i.e. Hexane, Ethanol, Ethyl-acetate and Isopropanol were purchased from Merck. Red tomatoes were obtained from local market and they were floated in the boiling water for 2min, and then they were cooled so that their tissues could be cut more easily. All samples must be kept in the cold and unlighted condition.Take 1gr of tissues in to 250 ml flask; add 30 ml of the solvents. The mixtures were stirred magnetically with 1000 rpm at room temperature for 30 min. Lycopene content in the extract was determined according to the absorbance measured at ~440, ~470, and ~500 nm relating to the three isomers of Lycopene (Fig. 1) via UV-Vis spectrophotometer (GBC cintra40).
3. Results and Discussion
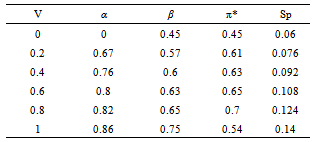
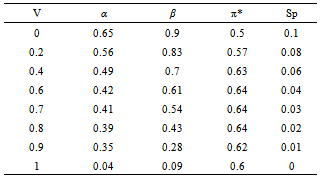
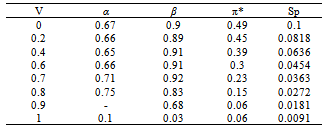
- Tables 1-3 and Figures 2-4 show the Sp, solvatochromic parameters and Absorption variation in Hexane/ Isopropanol, Ethyl-acetate/Ethanol and Benzene/Isopropanol mixtures. Solvophibicity is the consequence of increased hydrophobic interactions caused by a solute molecule that enforces the water structure. The values of Sp for many binary mixtures give a fair linear correlation with the volume fraction of constituents[15,16]. Thus, we have estimated the Sp values for Ethyl-acetate/Ethanol, Isoropanol/Hexane and Isoropanol/Benzene mixtures by the following equation (Eq. (1)):
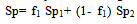 | (1) |
|
|
|
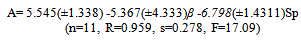 | (2) |
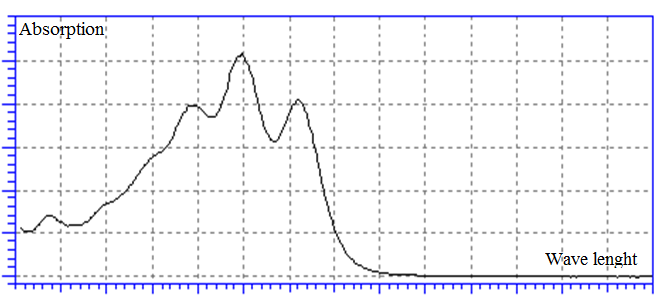 | Figure 1. UV-Vis spectrum of Lycopene |
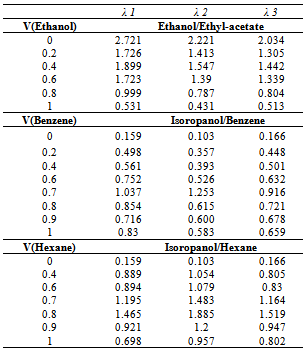
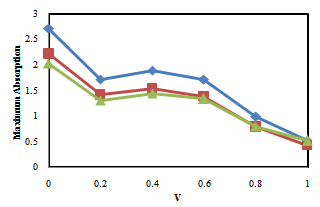 | Figure 2. Maximum Absorption versus Volume fraction in Ethanol/Ethyl-acetate mixtures. (♦) in λ=473 nm, (■) in λ=504.5 nm |
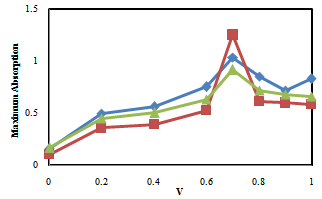 | Figure 3. Maximum Absorption versus Volume fraction inIsopropanol/Benzene mixtures. (♦) in λ=470.6 nm, (■) in λ=500.4 nmand (▲) in λ=445.8 nm. V is volume fraction of Benzene. |
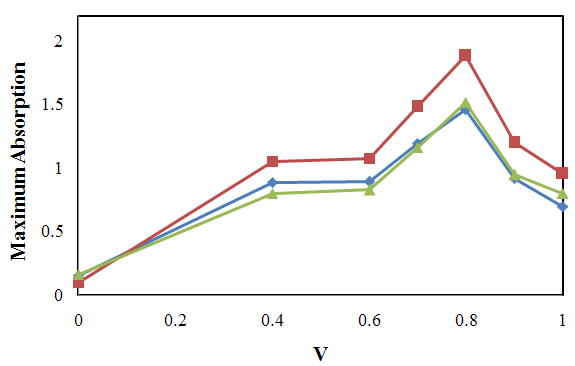 | Figure 4. Maximum Absorption versus Volume fraction in Isopropanol/Hexane mixtures. (♦) in λ=470.6 nm, (■) in λ=500.4 nm and (▲) in λ=445.8 nm. V is volume fraction of Hexane. |
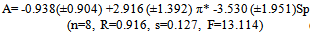 | (3) |
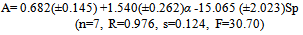 | (4) |
|
|
4. Conclusions
- Our results about the solvent-solvent and solute-solvent interaction in the Lycopene extraction process by three binary mixtures show:1- In Ethanol/Ethyl-acetate mixture Sp and β show the best correlation. By increasing the Ethanol fraction the solvent-solvent interaction increase causes the Lycopene extraction to decrease.2- Extraction in the Isopropanol/Hexane, Absorption had the best agreement with Sp and α parameters. The maximum extraction point and α parameter in the 80% of Hexane represented the more effect of the α parameter in extraction in this mixture.3- In the Isopropanol/Benzene, Sp and π* with negative and positive coefficients showed the best correlation versus extraction. The maximum extraction and the π*parameter in the 70% of benzene show the new complex of Isopropanol with more π*- π* interactions between this complex and Lycopene.
Acknowledgements
- The authors would also like to thank the Language Center for Sharif University of Technology-Kish International Campus, Especially Mr. M. Fardin, for the painstaking job of editing this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML