-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(2): 27-29
doi: 10.5923/j.chemistry.20120202.06
Ab-initio Study of the Ground State Structure and Properties of Fe+2 (Adenine)2 (H2O)2 Complex
D. De 1, S. Dhar 2, B. R. De 1
1Department of Chemistry and Chemical Technology, Vidyasagar University, Midnapore, 721102, India
2Department of Physics, Jhargram Raj College, Jhargram, Paschim Medinipur, India
Correspondence to: B. R. De , Department of Chemistry and Chemical Technology, Vidyasagar University, Midnapore, 721102, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
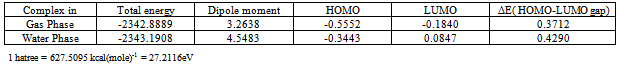
Hartree-Fock calculations with 6-31G(d) basis set have been done in the gas phase on Fe+2 (Adenine)2(H2O)2 complex with complete geometry optimization. Stable structure for the complex has been found. Single point water phase calculation (PCM) has also been done which shows that the complex is more stable in water implying its physiological action for the removal of excess hazardous Fe+2 from the body. Selected optimized geometrical parameters, charge densities on selected atoms have been reported. HOMO-LUMO energies and structures are shown. The LUMO structure shows that the Fe+2 play the key role of the complex. The study may help in the new drug discovery.
Keywords: Gaussian, Gas Phase, Water Phase, Hartree-Fock, Charge Density
1. Introduction
- The specific interactions between the purine and pyrimidine bases are one of the corner stones of the molecular biology[1-4]. It is well known[5] that hemoglobin provides an excellent illustration of quaternary structure. Each unit of hemoglobin is composed of α 2 and β2 chains. Hemoglobin is normally formed in the body. If any malfunctioning occurs in its formation[6] due to genetic or any other causes, several diseases like thalasemia, sickle cell anemia, excess Fe+2 accumulations etc. appear in the body. If this excess Fe+2 be removed by complexation with either of the purine or pyrimidine bases then there may be some possibilities of curing the above diseases. Previously chelate therapy was used to treat excessive quantities of copper in the body[6]. Keeping this view in mind the present work has been undertaken to examine theoretically the reaction between Fe+2 and adenine as the second case study because the complex with thymine has already been published[7].
2. Computational Details
- Complete geometry optimizations for Fe+2 (Adenine)2 (H2O)2 complex were done in the gas phase by Hartree-Fock method with 6-31G(d) basis set using Gaussian 03W program[8]. Actually this optimization gives the most stable conformation of the system studied. Moreover for first hand information Hartree-Fock method with 6-31G(d) basis set is sufficient in comparison to more popular DFT method which requires much more computation time. Single point water phase calculations at the gas phase equilibrium geometry were also done using the same basis set by polarisable continuum model (PCM) approach at HF level. Here also optimization in water phase calculation is not done because of computation time problem.
3. Results and Discussion
- Equilibrium geometry of the studied complex with the numbering scheme of the atoms is shown in figure-1 (a).The single point water phase calculations using the gas phase equilibrium geometry is shown in figure-1(b). The significant variations in these figures are indicated by some selective geometrical parameters given in Table-3. Table 1 reports in the gas phase the computed total energy (hartree), dipole moment (debye) and ΔE (HOMO-LUMO gap) (hartree), at the equilibrium geometry of the complex along with single point water phase data. Table-2 summarizes the computed net Mulliken charge on the selected hetero atoms of the complex at the equilibrium geometry of the complex in the gas phase including the single point water phase calculations. The hetero atoms are assumed to play the key role for the physiological drug action because these are the most negative centres of the complex. The drug action is assumed to take place through chemical attack.From the Table 1, it is seen that the complex is highly stable in the gas phase at the equilibrium geometry. The stability is increased in the water phase because the total energy of the system in this phase is more negative than in gas phase. Mulliken population analysis is not unique; still it is very important to provide an idea of electron density distribution in a molecular system. The calculated dipole moment reflects an overall charge/electron density distribution in a molecular system. From Table -1 it is seen that the dipole moment of the complex in water phase is 1.5 times greater than that in gas phase as it is expected [polar complex in polar solvent]. The HOMO and LUMO both are little bit destabilized in water phase than the gas phase because the complex contains ten N-atoms.From Table 2 it is seen that the Fe+2 (in its closed shell configuration) ion receives 0.5072e and 0.4493e amount of charge respectively in gas phase and water phase from two adenine ligands and two water ligands. In almost all the hetero atoms the charge densities in gas phase is little less than that in the water phase implying that in water phase the negative centers become more effective for better physiological drug action.HOMO and LUMO structures of the complex both in gas phase and single point water phase are shown in figure-( 2a, 2b )and figure- (3a, 3b) respectively. The significance of the figures is that complete geometry of the complex is seen which is not reflected from the selected geometrical parameters shown in Table-3. The LUMO structure shows that Fe+2 ion plays the key role of the complex both in gas phase and water phase which is also supported from figures. Table-3 shows some selected geometrical parameters of the complex in the gas phase particularly the metal containing zone and other heteroatom containing zone. From table-3 it is seen that Fe+2 containing zones are non planar and this part of the complex is out of plane from both the adenine ligands and two H2O ligands also. Fe31 –N25 and Fe31-N11 distances are 3.6100 A° and 3.2200A° respectively. Fe31-N18 and Fe31-N12 distances are 2.0662 A° and 2.0610A° respectively. Fe31-O36 and Fe31-O33 distances are 2.0185A° and 2.0089A° respectively. The most stable conformation of the system favors such geometrical parameters.
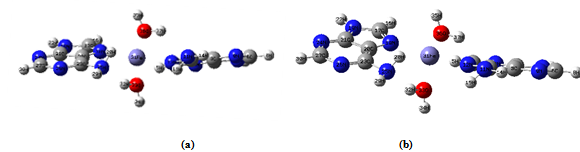 | Figure 1. (a) Optimized structure of Fe+2 (Adenine)2 (H2O)2 complex in gas phase. (b)Single point structure of Fe+2 (Adenine)2 (H2O)2 complex in water phase |
|
|
|
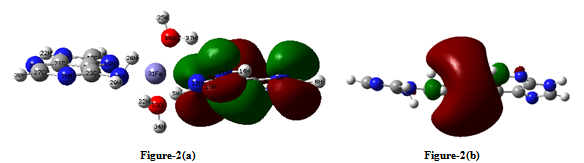 | Figure 2. (a) HOMO structure of Fe+2 (Adenine)2 (H2O)2 complex in gas Phase. (b) LUMO structure of Fe+2 (Adenine)2 (H2O)2 complex in gas Phase |
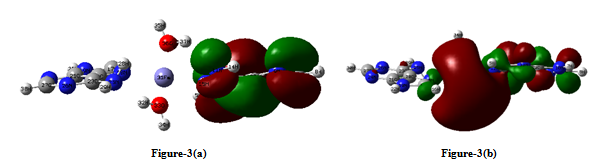 | Figure 3. (a) HOMO structure of Fe+2 (Adenine)2 (H2O)2 complex in water Phase. (b) LUMO structure of Fe+2 (Adenine)2 (H2O)2 complex in water Phase |
4. Conclusions
- From the present theoretical study it is found that the Fe+2 (Adenine)2 (H2O)2 Complex is a stable complex both in gas phase and in water phase (single point calculation by polarisable continuum model- PCM). This first hand information of the title complex signifies its physiological importance for the removal of excess Fe+2 from the body. The LUMO structure shows that Fe+2 ion plays the key role of the complex both in gas phase and water phase. The present study may help in the discovery of new drug.
References
| [1] | Saengen W (1984) Principles of Nucleic Acid Structure, Springer Verlag, |
| [2] | Jeffrey G.A et.al (1991) Hydrogen Bonding in Biological structures, Springer Verlag, Berlin, Heidelberg, Newyork |
| [3] | Huysken Th Zeegers, et.al (1991) In intermolecular forces. An introduction to Modern Methods and Results Springer Verlag, Berlin, Heidelberg, Newyork 24 |
| [4] | Chandra A. K. et.al (1998) J. Phys. Chem. A 102 6010-6016. |
| [5] | Snustad D.P. et.al (2003) Principles of Genetics, third edition, John Willey and Sons, 303-307 |
| [6] | J.E Huheey J.E et.al (2004) Inorganic Chemistry, Principles of structure and reactivity, Sixth edition, Pearson Education 902-908, 956-960 |
| [7] | De D., Dalai S., De B.R.(2011) Ab-initio Study of the Ground State Structure and Properties of Fe+2 (Thymine)2 (H2O)2 Complex, AJPAC,5(9) ,293-296 |
| [8] | (a) Frisch M.J. Gaussian 03W Program (Gaussian, Inc., Wallingford, CT) 2004 (b) Lee,C (1988) Phys Rev B 37 785 (c) Becke A D et.al (1993) J Chem Phys 98 5648 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML