| [1] | Zhibo Yang, "Experimental and theoretical studies noncovalent metal ligand complexes :applications to solvation and nucleobase tautmerization and modification" , Ph.D. thesis, Wayne State University, Detroit, Michigan, USA., 2005. |
| [2] | Gy. Jakli, "Evaluation of individual ionic partial molar volumes in aqueous solutions" , Journal of Chemical Thermodynamics, vol.40, pp.770-776, 2008. |
| [3] | Yizahak Marcus, "On the preferential salvation of drugs and PAHs in binary solvent mixtures" , Journal of Molecular Liquids,vol.140, pp. 61-67, 2008. |
| [4] | Krishna Rajagopal, Edwin Gladson, "Partial molar volume and partial molar compressibility of four homologous alpha amino acids in aqueous sodium fluoride solution at different temperatures" , Journal of Chemical Thermodynamics, vol.43, pp. 852-867, 2011. |
| [5] | Sk M Ali. Dlip K Maity, Sulagno De, M R K Shenoi, "Ligands for selective metal ion extraction: A molecular modeling approach" , Deslination, vol.232, 181-190, 2008. |
| [6] | Hongwei Qias, Helin Luan, Zhiming Zhou, Lxue Bi, Wen Yao, Jimei Li, Cheng Chen, "Vibrational spectroscopic and density functional theory studies on ion salvation and ion association of lithium tetraphenylcarbonyl chloride-based solvents" , Journal of Molecular structure, vol.885, pp. 89-96, 2008. |
| [7] | Rakesh K. Mahajan, Arifa Shahees, "Effect of various additives on the performance of newly developed PVC base potentiometric sensor for anion surfactants" , Journal of Colloid and Interface Science, vol.326, pp. 191-195, 2008. |
| [8] | Albert J Fry , Lawrence K Steffen, "On the nature of tetraalkylammonium ions in common electrochemical solvents : General and specific salvation-Quantitative aspects" , Journal of Electroanalytical Chemistry,638(2010)218-224. |
| [9] | Esam A Gomaa, Badria. M Al Jahdali, "Association of Cu(NO3)2 with Kryptofix-221 in mixed (MeOH-DMF) solvents at different temperatures" , American Journal of Fluid Dynamics, vol. 1(1), pp. 4-8, 2011. |
| [10] | Nagah El-Shishtawi, Maany A Hamada, Essam A Gomaa, "Inflence of Permanent magnet on the association constants of FeCl3+10% PVA(Polyvinylalcohol) in50% ethanol-water solutions conductometrically at 298.25K using new equation for 1:3 asymmetric electrolytes" , Physical Chemistry, vol.1(1), pp.14-16, 2011. |
| [11] | Esam A Gomaa, "Solvation parameters of lead acetate in mixed N,N-dimethylformamide-water mixtures at 298.15K" , Analele Universitatii din Bucuresti-Chimie, vol.19, pp.45-48. 2010. |
| [12] | Waclaw Gryzybkowski, Michael Pilarczyk, "Electricalconductance and apparent molar volumes ofAl(ClO4)2,Be(ClO4)2 and Cu (ClO4)2 in N,N-dimethylformamide solutions at 25℃" , Electrochimica Acta, vol.32, pp.1601-1605, 1989. |
| [13] | Nagah A El-Shishtawi, Maany A Hamada, Esam A Gomaa, "Influence of permanent magnet on the association constants of FeCl3 in 50% ethanol-H2O solutions (conductometrically) at 298.15 K using a new equation for 1:3 asymmetric electrolytes" , Journal Chemical engineering data, vol.55, pp.5422-5424, 2010. |
| [14] | Maany A Hamada, Nagah A El-Shishtawiand, Essam A Gomaa, "Conductometric evaluation of association constants for aqueous solutions of CoCl2 in the absence and presence of a magnetic field" , Southern Brazilian journal of chemistry, vol.17, pp. 33-40, 2009. |
| [15] | Esam A Gomaa, "Solute-solvent interactions of some univalent-univalent salts with various organic solvents at 25℃" , Thermochimica Acta, vol.120, pp.183-189, 1987. |
| [16] | Esam A Gomaa, "Theoretical contribution of solvation of AgBr in some organic solvents at 25℃" , Thermochimica Acta, vol.128, pp. 99-120, 1988. |
| [17] | Farid I. El-Dossoki, "Electric conductance andsemi-emperical studies on two thiophene derivatives/metal cation complexation, Journal of Molecular Liquids, vol.142, pp.53-56, 2008. |
| [18] | M. Rahmi-Nasrabadi, F. Ahmedi, S. M. Pourmor-tazari, M. R. Ganjal, Kamal Alizadeh, "Conductometric study of complex formation between some substituted pyrimidines and some metal ions in acetonitrile and the determination of thermodynamic parameters" , Journal of Molecular Liquids, vol.144, pp.97-101, 2009. |

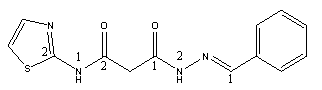
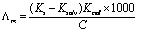
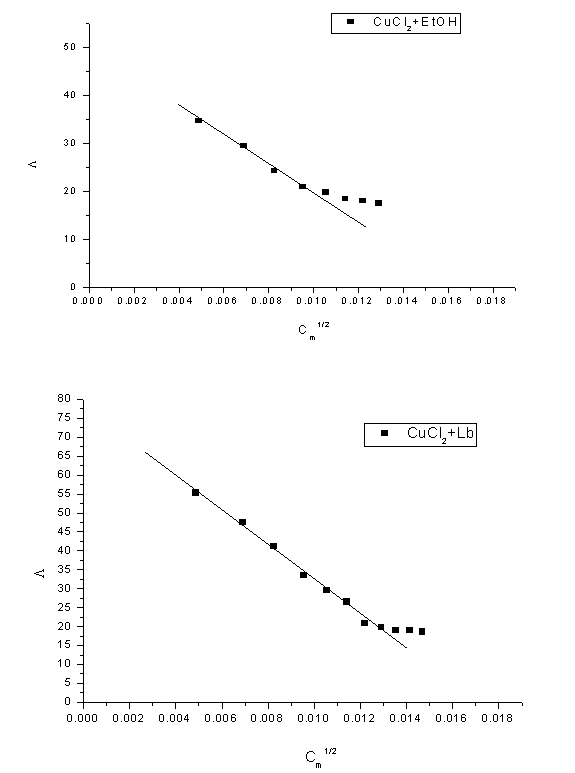
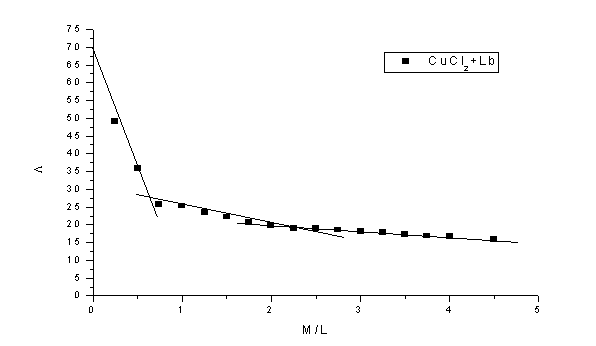
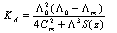
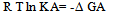
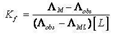
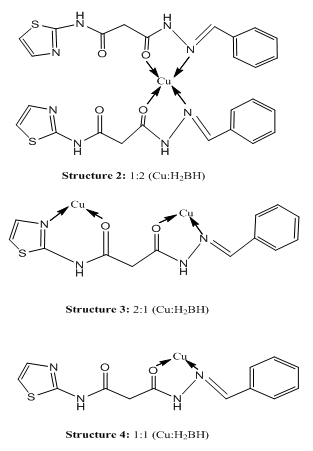
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML