-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(2): 12-17
doi: 10.5923/j.chemistry.20120202.03
Kinetics and Mechanism of Oxidative Conversion of 5-Hydroxyindole to 2-Oxo-5-hydroxyindole by Chloramine-B in Basic Solutions
Nirmala Vaz 1, K. N. Vinod 2, Puttaswamy 2, Netkal M. Made Gowda 3
1Department of Chemistry, Jyoti Nivas College, Hosur Road, Bangalore, 560095, India
2Department of Post-Graduate Studies in Chemistry, Central College, Bangalore University, Bangalore, 560001, India
3Department of Chemistry, Western Illinois University, 1 University Circle, Macomb, IL 61455, USA
Correspondence to: Netkal M. Made Gowda , Department of Chemistry, Western Illinois University, 1 University Circle, Macomb, IL 61455, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The conversion of 5-hydroxyindole (Indole) to 2-oxo-5-hydroxyindole is an important oxidative transformation in synthetic organic and biochemistry. Under pseudo first-order conditions of[Indole]o >>[chloramine-B or CAB]o, the indole oxidation reaction in a basic solution follows the following experimental rate law: rate = k‘[CAB]o[Indole]o[OH-]x/[BSA]y, where BSA represents benzenesulfonamide and x and y are fractional orders. The retarding effect of BSA on the rate indicates its involvement in a fast pre-equlibrium in the mechanism of oxidation. Activation parameters have been determined. The variation of[OH-] at constant[BSA] was performed to evaluate the equilibrium and decomposition constants. Based on the mechanism proposed, a rate law has been derived. Furthermore, the methodology developed could be adopted in the synthesis of 2-oxo-5-hydroxyindole. Advantages of the method include: mild reaction conditions, short reaction time, ease of isolation of products, cost effectiveness, and the use of eco friendly reagents.
Keywords: 5 – Hydroxyindole, 2 - Oxo - 5 – Hydroxyindole, Chloramine – B, Benzenesulfonamide, Oxidation Kinetics, Mechanism
1. Introduction
- Heterocyclic compounds such as indoles are known for their various biological activities and some of them are pharmaceutically important[1]. The oxidative metabolism of indoles in cells is an essential pathway for their detoxification[2]. The oxidation of indoles leads to a number of products depending on the nature of substituents on the indole nucleus and more particularly on the pyrrole moiety. Indoles, which are metabolites of the neurotransmitter, serotonin, found in brain act as precursors[3]. Furthermore, the nature of oxidizing agents and reaction conditions play an important role in the oxidation of indoles[4-5]. The oxidation of indoles to the oxoindole stage is an important transformation in organic as well as in biochemistry[6]. There are several methods available in the literature for the conversion of indoles to oxoindoles; but they are complex in nature and require vigorous conditions[4-5]. Mechanisms of oxidation of 5-substituted indole derivatives (viz, 5-H, -OCH3, -Br and –Cl) to the corresponding oxoindoles by alkaline chloramine-T and chloramine-B have been studied kinetically in the presence of OsO4 catalyst[7-8]. The presence of hydroxyl group at position-5 in indole makes it biologically significant as it plays a vital role in functions of the central nervous system (CNS)[9]. The literature survey indicates that there is no report on the oxidation of 5-hydroxyindole by aromatic N-haloamines from the viewpoint of its kinetic and mechanistic aspects. In the present kinetic study, 5- hydroxyindole is oxidized to 2-oxo-5-hydroxyindole, which is biologically and pharmaceutically important. It is also a precursor in the synthesis of natural products[10]. Organic sulfonyl-N-haloamines are mild oxidants containing a strongly polarized N- bonded halogen in its +1 oxidation state. Kinetics and mechanisms of oxidation by these reagents have attracted the attention of chemists due to their diverse properties to act as halonium cations (X+), hypohalites and N-anions[11]. They interact with a wide range of functional groups in aqueous, partially aqueous, and non-aqueous media in the presence of an acid or a base. As a result, these reagents have been used as mild and selective oxidizing agents in synthetic organic chemistry[12]. The prominent member of this group, sodium N-chloro-p- toluenesulfonamide, commonly known as chloramine-T (CAT), is a known analytical reagent and the kinetic and mechanistic aspects of many of its reactions have been well documented[12-16]. Chloramine-B is the benzene analog of CAT, which can be easily prepared from the reaction of benzenesulfonamide and chlorine in basic solutions[17]. Chlora- mine-B is a stable compound with slightly higher active chlorine content than CAT. It liberates iodine from acidified aqueous KI solutions and undergoes two-electron reductions to form benzenesulfonamide and sodium chloride. Though the literature shows several reports on the kinetic and mechanistic aspects of CAT, a similar information with CAB is lacking[18-20]. Our preliminary experiments have revealed that CAB acts as an efficient oxidant as compared to CAT for the smooth and efficient conversion of 5-hydroxyindole to 2-oxo-5-hydroxyindole in basic solutions. Hence, the investigation on the indole-CAB redox reaction has been chosen in the present work. Main objectives of the present study are to: (i) determine reaction stoichiometry and characterize the oxidation products; ii) determine the kinetic and activation parameters; (iii) elucidate a plausible mechanism; (iv) derive a consistent kinetic rate law; (v) ascertain the reactive species; and (vi) optimize the reaction conditions for the efficient conversion of 5-hydroxyindole to 2-oxo-5-hydroxyindole, which can be used as a method in organic synthesis. The latter methodology has several advantages including the simplicity with short reaction time, ease of isolation of products, cost effectiveness, and the use of relatively environmentally benign reagents.
2. Experimental
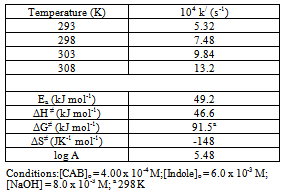
- The purity of CAB (Aldrich Chemical Co.) was checked by determining the amount of active chlorine present in the compound. A stock aqueous solution of CAB was standardized iodometrically and preserved in brown bottles to prevent its photochemical deterioration[21]. An aqueous solution of 5-hydroxyindole (Aldrich Chemical Co.) was prepared and used. Heavy water (D2O, 99.2%) was obtained from Bhabha Atomic Research Center (Mumbai, India). All other reagents used were of analytical grades of purity. Double distilled water was used in all solutions. Kinetic procedureReactions were carried out under pseudo-first order conditions, ([Indole]o >>[CAB]o), in stoppered Pyrex glass boiling tubes whose outer surface was coated black to prevent photochemical effects. Appropriate amounts of solutions of 5-hydroxyindole and NaOH and water (to keep the total volume of 50 mL constant for all runs), were taken in the boiling tube and thermostated at 25 ± 0.1 0C. A measured amount of CAB solution, also thermosated at the same temperature, was rapidly added with stirring to the mixture in the boiling tube. The progress of the reaction was monitored by withdrawing 5-mL aliquots from the reaction mixture at different time intervals and iodometrically determining the unreacted CAB concentration using a standard thiosulfate solution with starch indicator. The course of the reaction was followed for two half-lives. Pseudo-first-order rate constants, k/, calculated from plots of log (titer) or[CAB] vs. time were reproducible within ±5%.
3. Results
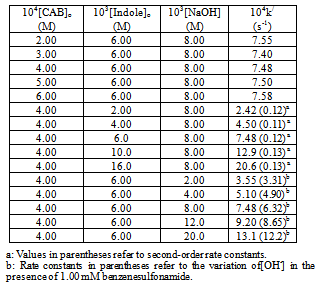
- Stoichiometry. Reaction mixtures with varying CAB-to-indole compositions in the presence of 8.0 mM NaOH were equilibrated at 298 K for 24 h. The iodometric determination of the residual oxidant showed that one mole of indole consumed one mole of CAB. The 1:1 stoichiometry obtained can be represented as in eq. (1).
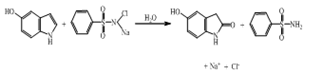 | (1) |
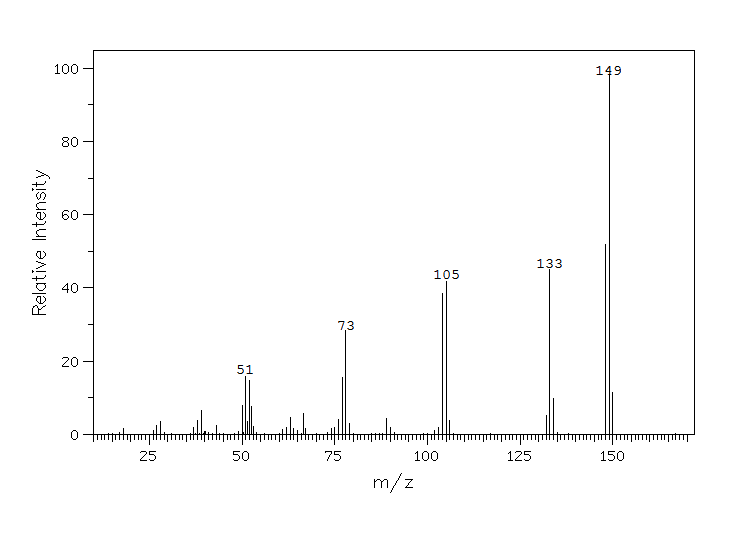 | Figure 1. GC-MS spectrum of 2-oxo-5-hydroxyindole with a parent molecular ion peak at 149 amu. |
|
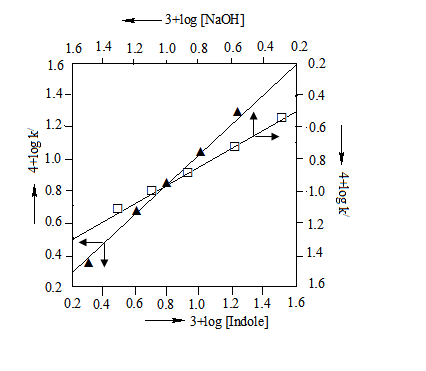 | Figure 2. Plots of log k/ versus log[Indole] and log k/ vs. log[NaOH]. Experimental conditions are the same as in Table 1. |
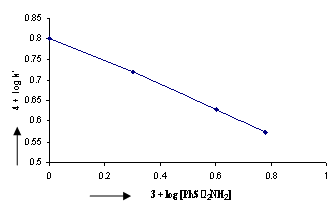 | Figure 3. A Plot of log k/ vs. log[PhSO2NH2]. Experimental conditions:[CAB]o = 4.00 x 10-4 M;[Indole]o = 6.00 x 10-3 M;[NaOH] = 8.00 x 10-3 M; T = 298 K. |
|
4. Discussion
- Reaction mechanism In general, both CAB and CAT, which undergo a two-electron change in their reactions, exhibit similar equilibria in aqueous solutions[23]. The redox potential of the CAB/PhSO2NH2 system is dependent on pH of the medium. Chloramine-B in aqueous solutions behaves as a strong electrolyte and, depending on the pH of the medium, it furnishes the following species in several equilibria[21,23-25]:
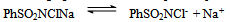 | (2) |
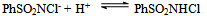 | (3) |
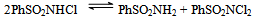 | (4) |
 | (5) |
 | (6) |
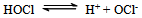 | (7) |
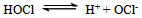 | (8) |
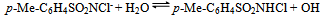 | (9) |
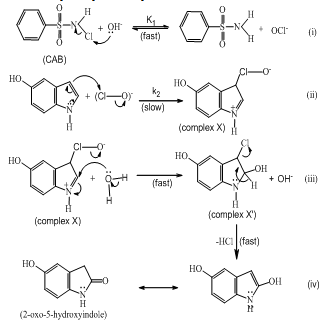 | Scheme 1. Detailed mechanistic scheme for the oxidation of 5- hydroxyindole by CAB in basic solutions. |
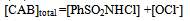 | (10) |
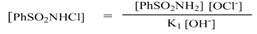 | (11) |
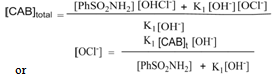 | (12) |
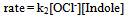 | (13) |
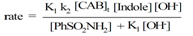 | (14) |
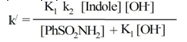 | (15) |
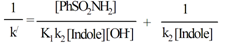 | (16) |
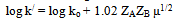 | (17) |
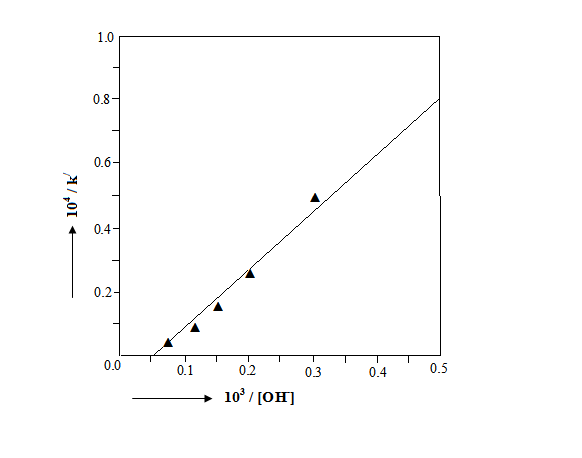 | Figure 6. A plot of 1/k/ vs. 1/[OH-]. Experimental conditions:[CAB]o = 4.00 x 10-4 M;[Indole]o = 6.00 x 10-3 M;[PhSO2NH2] = 1.0 x 10-3 M; T = 298 K. |
5. Conclusions
- The kinetics of oxidation of 5-hydroxyindole by CAB in basic medium follows the experimental rate law: -d[CAB]/dt =[CAB]o[Indole]o[OH-]0.55/[BSA]0.33. A suitable mechanism resulting in a derived rate law consistent with the experimental data is suggested. The present method for the oxidative conversion of 5-hydroxyindole to2-oxo-5-hydroxyindole has the following advantages: simplicity of the method with a short reaction time and the use of cost-effective and environmentally benign reagents. Furthermore, this method could be adopted for the conversion of 5-hydroxyindole to 2-oxo-5-hydroxyindole in organic synthesis.
ACKNOWLDGEMENTS
- One of the authors (NV) is grateful to the University Grant Commission, New Delhi, for awarding a minor research project (MRP (s) 003/07-08/KABA021/UGC-SWRO) and thanks the Principal and Management of Jyoti Nivas College for encouragement. Authors also thank Prof. M. A. Pasha for helpful suggestions on the oxidation mechanism.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML